Answer: (9, 9)
Step-by-step explanation:

Answer:
<h2><em><u>Obtuse</u></em><em><u> </u></em><em><u>Angle</u></em><em><u> </u></em></h2>
Step-by-step explanation:
An obtuse angle has a measurement greater than 90 degrees but less than 180 degrees.
2000x4=8,000.
8,000+4000=12,000.
2,000 per hour. u multiply per hour they need. then add 4,000 for the basic fee. ur total is 12,000.
Answer:
(pound sign) 11.44
Step-by-step explanation:
Answer:
Null Hypothesis, 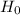 : p
: p  85%
85%
Alternate Hypothesis,  : p > 85%
: p > 85%
Step-by-step explanation:
We are given that it's been found that 85% of American adults suffer from triskaidekaphobia (fear of the number 13).
A USA Today/Gallup poll asked 1,006 randomly selected people 18 years old and older in telephone interviews "Suppose you checked into a hotel and were given a room on the thirteenth floor. Would this bother you or not?" 87% of the survey respondents answered they would be bothered.
Let p = <u><em>proportion of respondents who would be bothered about given a room on the thirteenth floor</em></u>
SO, Null Hypothesis, 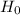 : p
: p  85%
85%
Alternate Hypothesis,  : p > 85%
: p > 85%
Here, <u>null hypothesis states that</u> the proportion of respondents who would be bothered about given a room on the thirteenth floor is less than or equal to 85%.
On the other hand, <u>alternate hypothesis states that</u> the proportion of respondents who would be bothered about given a room on the thirteenth floor is more than 85%.
Hence, this would be the correct null and alternative hypothesis.