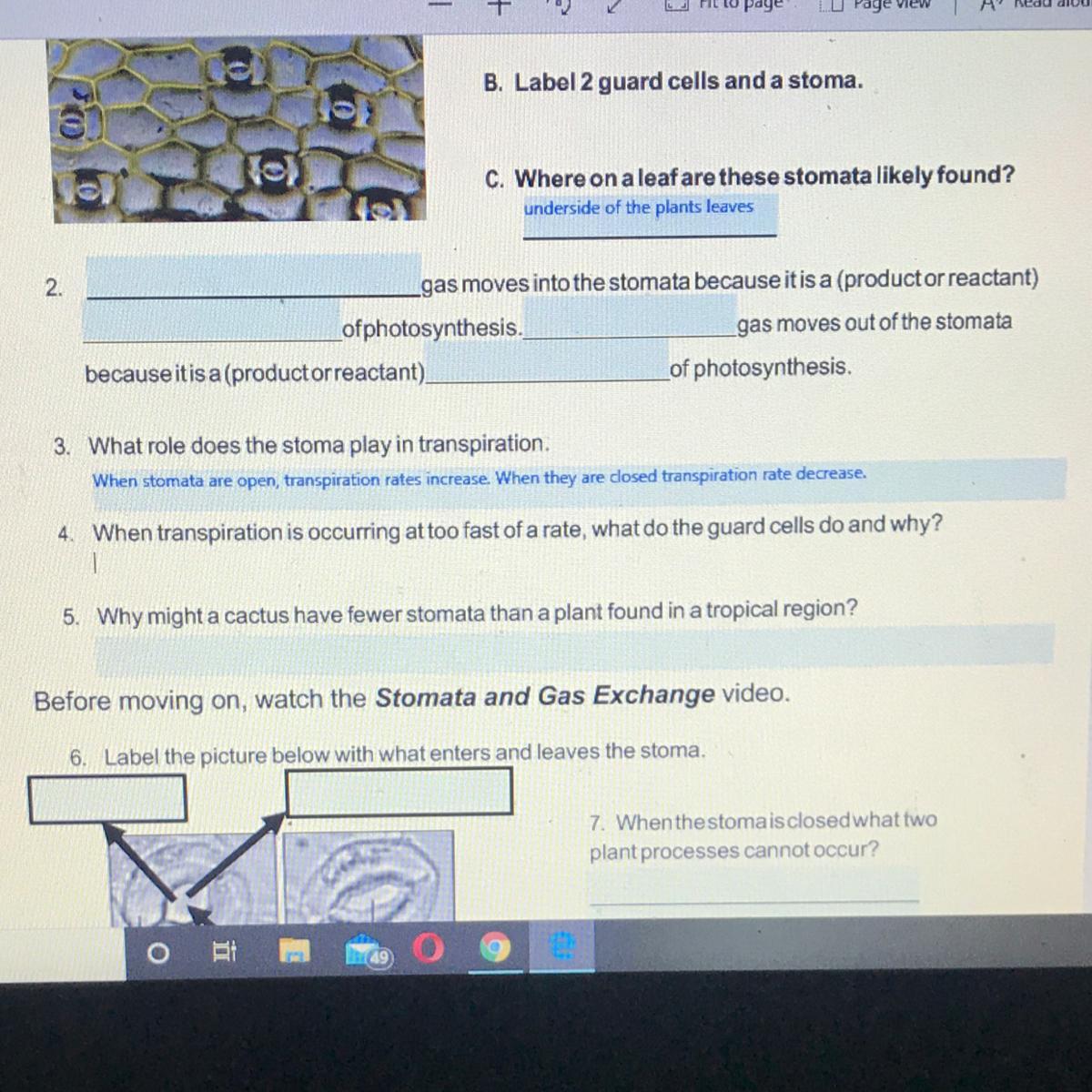
Answer:
© CK-12 (Middle School)
Terms of Use
Licensed under
Questions have been modified from original.
ASSIGNMENT DIRECTIONS
As you read, think about the following: How can the excretory system be compromised?

1
Why drink water?
2
It's always a good idea to drink plenty of fluids, especially when you have been exercising. Drinking plenty of water helps to flush away materials that might form kidney stones. Staying hydrated is the best way to prevent kidney stones.
Problems of the Excretory System
3
The urinary system controls the amount of water in the body and removes wastes. Any problem with the urinary system can also affect many other body systems.
Kidney Stones
4
In some cases, certain mineral wastes can form kidney stones (Figure below). Stones form in the kidneys and may be found anywhere in the urinary system. Often, stones form when the urine becomes concentrated, allowing minerals to crystallize and stick together. They can vary in size, from small stones that can flow through your urinary system, to larger stones that cannot. Some stones cause great pain, while others cause very little pain. Some stones may need to be removed by surgery or ultrasound treatments.
5
A kidney stone. The stones can form anywhere in the urinary system.
6
What are the symptoms of kidney stones? You may have a kidney stone if you have pain while urinating, see blood in your urine, and/or feel a sharp pain in your back or lower abdomen (the area between your chest and hips). The pain may last for a long or short time. You may also have nausea and vomiting with the pain. If you have a small stone that passes on its own easily, you may not experience any symptoms. If you have some of these symptoms, you should see your doctor.
QUESTION 1 DOK 3 NO STANDARD
Why does the author argue that "staying hydrated is the best way to prevent kidney stones?"
EXTRA HELP
What are kidney stones?
When do they form: When a person has drank a lot of water or not enough water?
Kidney failure
7
Kidney failure happens when the kidneys cannot remove wastes from the blood. If the kidneys are unable to filter wastes from the blood, the wastes build up in the body. Kidney failure can be caused by an accident that injures the kidneys, the loss of a lot of blood, or by some drugs and poisons. Kidney failure may lead to permanent loss of kidney function. But if the kidneys are not seriously damaged, they may recover.
8
Chronic kidney disease is the slow decrease in kidney function that may lead to permanent kidney failure. A person who has lost kidney function may need to get kidney dialysis. Kidney dialysis is the process of filtering the blood of wastes using a machine. A dialysis machine (Figure below) filters waste from the blood by pumping the blood through a fake kidney. The filtered blood is then returned to the patient’s body.During dialysis, a patient’s blood is sent through a filter that removes waste products. The clean blood is returned to the body.
QUESTION 2 DOK 2 NO STANDARD
Why is kidney dialysis a solution for patient's with kidney failure?
EXTRA HELP
What is kidney failure?
What does a dialysis machine do? How does this help a patient with kidney failure?
Urinary tract infections (UTIs)
9
Urinary tract infections (UTIs) are bacterial infections of any part of the urinary tract. When bacteria get into the bladder or kidney and produce more bacteria in the urine, they cause a UTI. The most common type of UTI is a bladder infection. Women get UTIs more often than men. UTIs are often treated with antibiotics.
10
Most UTIs are not serious, but some infections can lead to serious problems. Long lasting kidney infections can cause permanent damage, including kidney scars, poor kidney function, high blood pressure, and other problems. Some sudden kidney infections can be life threatening, especially if the bacteria enter the bloodstream, a condition called septicemia.
11
What are the signs and symptoms of a UTI?
- a burning feeling when you urinate,
- frequent or intense urges to urinate, even when you have little urine to pass,
- pain in your back or side below the ribs,
- cloudy, dark, bloody, or foul-smelling urine,
- fever or chills.
12
You should see your doctor if you have signs of a UTI. Your doctor will diagnose a UTIs by asking about your symptoms and then testing a sample of your urine.
QUESTION 3 DOK 1 NO STANDARD
What is a UTI and why is it problematic?
EXTRA HELP
What is a UTI? Refer back to paragraph 9.
What is problematic about UTIs? Refer back to paragraph 10.
Summary
Disorders of the urinary system include kidney stones, kidney failure, and urinary tract infections.
Kidney dialysis is the process of filtering wastes from the blood using a machine.
Explore More