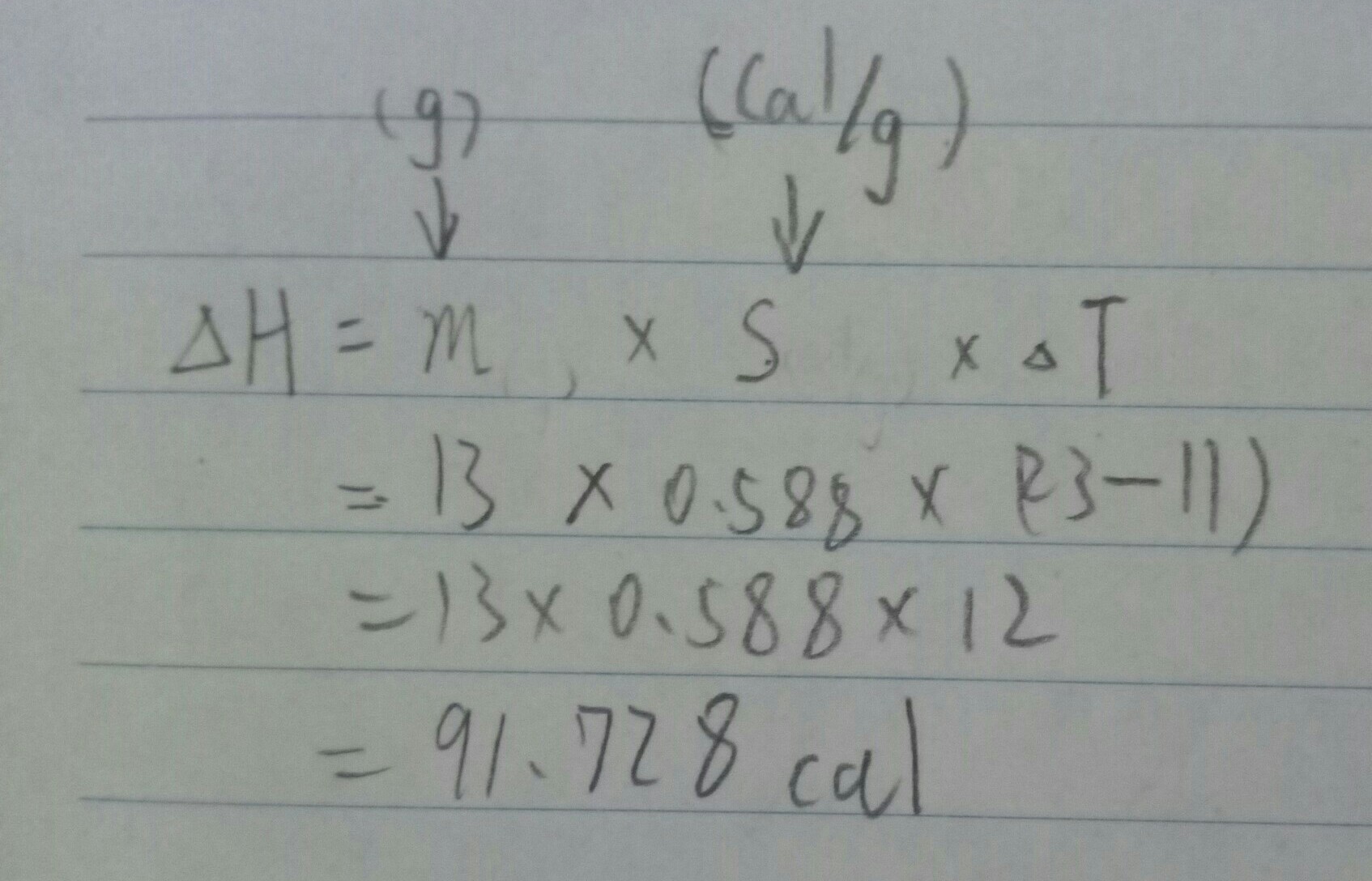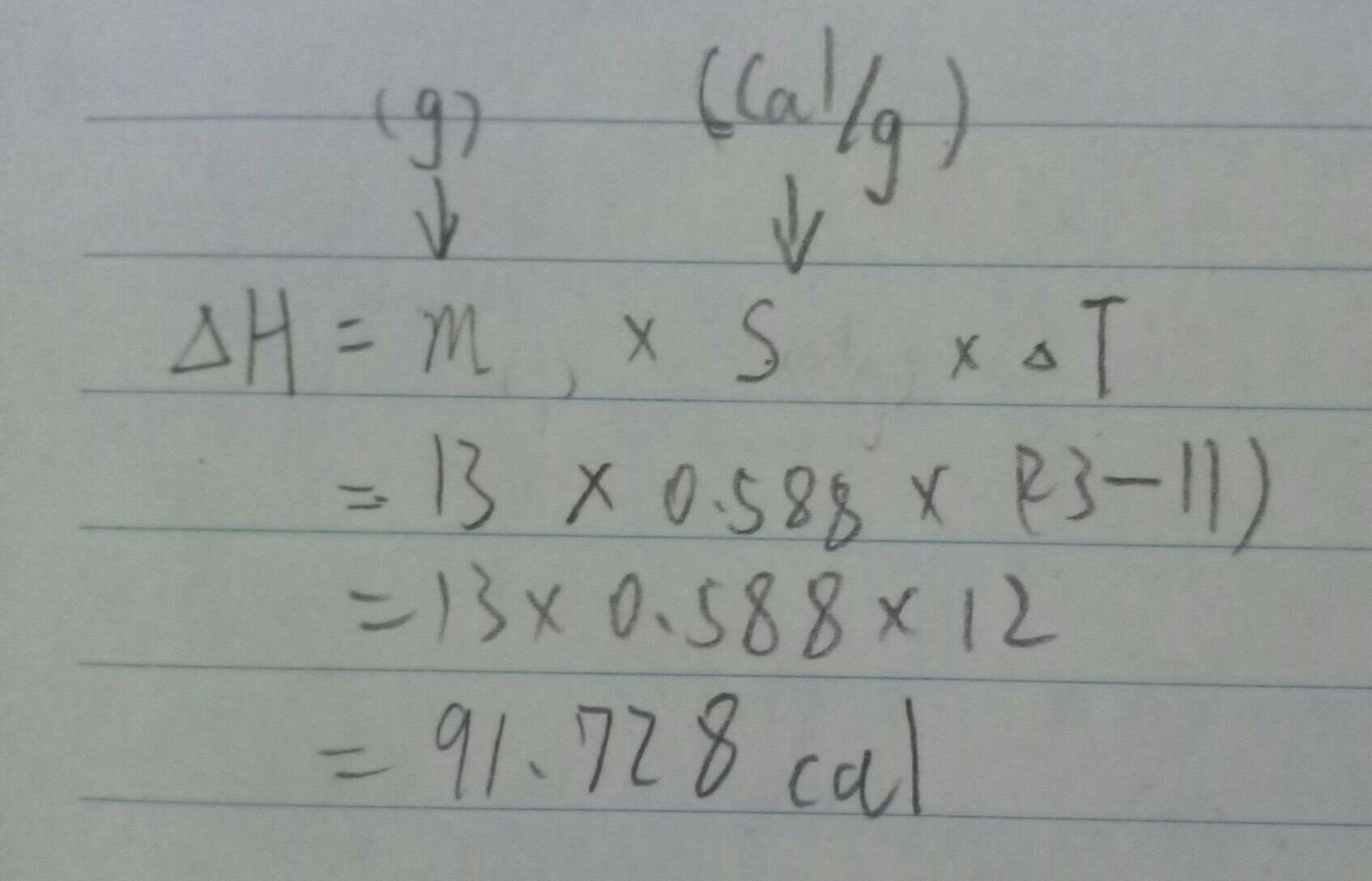Answer:
Explanation:
1. The element you selected is Sodium. some of the physical properties are
i. Sodium has a strong metallic lustre
ii. Sodium are usually silver white in color
iii. Sodium is soft it can be cut with knife
iv. Sodium are malleable and ductile.
v. Sodium conduct electricity
Sodium belong to group 1 elements which are called Alkali metals. Sodium have one valency electron and it gives this electron easily during bonding . This is one reason sodium and other alkali metals are very reactive. Sodium can instantly be oxidized by water. Sodium has a relative atomic mass of approximately 23 amu.
2. There are various models that describe atoms .This models includes John Dalton models, J.J Thompson model, Ernest Rutherford model, Bohr's models etc. This models made us understand the role and location of each sub atomic particles. The models shed more light on subatomic particles like electron which revolve around the nucleus. It provide more information on what contribute to the weight of an atom.
3. The proton , neutron and electron are subatomic particles of an atom. The proton and the neutron are located at the nucleus of an atom. The proton and neutron contributes majorly to the weight of an atom. The proton is positively charge while neutron has no charge.
Electron is found to move around the nucleus in cloud. The electron is negatively charge. The electron determines bonding of atoms. An atom is electrically neutral if the number of proton is equal to the number of electron.
4. The four fundamental forces includes weak nuclear forces , strong nuclear forces , gravity and electromagnetic forces.
Weak nuclear forces is responsible for particle decay .Nuclear forces don't play a role in chemical reaction . If they are involve in a reaction it becomes nuclear reaction not chemical reaction.
Strong nuclear forces causes a strong nuclear interaction and it is the strongest among the 4 fundamental forces. This strong force only operate when subatomic particles are much closer to each other.
Gravity force is too weak to affect chemical reaction much. The gravity forces involves the interaction between two objects with energy and mass.
The electromagnetic force act between charged particles like negatively charged electron and positively charged proton. Electromagnetic force is the most important force in chemical bonding as it depends on the arrangement of atoms and the state of their electron. Electromagnetic forces exist in various forms like the covalent bond, metallic, ionic , dipole dipole, hydrogen bonds and many more. The major force involve in chemical bonding is the electromagnetic force.