Answer:
We conclude that there has been a decrease in the percentage of females in the company from last year.
Step-by-step explanation:
We are given that Last year, 50% of MNM, Inc. employees were female. It is believed that there has been a reduction in the percentage of females in the company.
This year, in a random sample of 400 employees, 180 were female.
<u><em /></u>
<u><em>Let p = percentage of females in the company this year.</em></u>
So, Null Hypothesis, 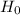 : p
: p  50% {means that there has not been a decrease in the percentage of females in the company from last year}
50% {means that there has not been a decrease in the percentage of females in the company from last year}
Alternate Hypothesis,  : p < 50% {means that there has been a decrease in the percentage of females in the company from last year}
: p < 50% {means that there has been a decrease in the percentage of females in the company from last year}
The test statistics that will be used here is <u>One-sample z proportion</u> <u>statistics</u>;
T.S. =  ~ N(0,1)
~ N(0,1)
where,  = proportion of female in a sample of 400 employees =
= proportion of female in a sample of 400 employees =  = 0.45
= 0.45
n = sample of employees = 400
So, <u><em>test statistics</em></u> = 
= -2.0101
The value of the test statistics is -2.0101.
Now at 5% significance level, the z table gives critical value of -1.6449 for left-tailed test. Since our test statistics is less than the critical value of z as -2.0101 < -1.6449, so we have sufficient evidence to reject our null hypothesis as it will fall in the rejection region due to which <u>we reject our null hypothesis</u>.
Therefore, we conclude that there has been a decrease in the percentage of females in the company from last year.