Answer:
Yes, FDA should reject the drug at 5% significance level.
Step-by-step explanation:
We are given that a new drug is being proposed for the treatment of migraine headaches. The FDA will reject the drug if it thinks that more than 15% (i.e. 0.15) of the population would suffer from this side effect.
Let, NULL HYPOTHESIS, 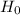 : p
: p  15% {means that the % of population that suffer from this side effect is less than or equal to 15%}
15% {means that the % of population that suffer from this side effect is less than or equal to 15%}
ALTERNATE HYPOTHESIS, 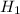 : p > 15% {means that the % of population that suffer from this side effect is more than 15%}
: p > 15% {means that the % of population that suffer from this side effect is more than 15%}
Also, in an experiment to test this side effect, 400 people who suffer from migraine headaches receive the new drug and 80 of them report nausea as a side effect.
The test statistics that will be used here is One-sample z proportion test;
T.S. =  ~ N(0,1)
~ N(0,1)
where,  = proportion of people who suffer from migraine headaches in an
= proportion of people who suffer from migraine headaches in an
experiment of 400 people =  = 0.20
= 0.20
n = sample of people = 400
So, test statistics = 
= 2.50
<em>Now, at 5% significance level z table gives critical value of 1.6449. Since our test statistics is more than the critical value of z so we have sufficient evidence to reject null hypothesis as it will fall in the rejection region.</em>
Therefore, we conclude that % of population that suffer from this side effect is more than 15% which means FDA should reject the drug.