In new york, carbon monoxide levels shrank to half their usual in march! :)
Answer:
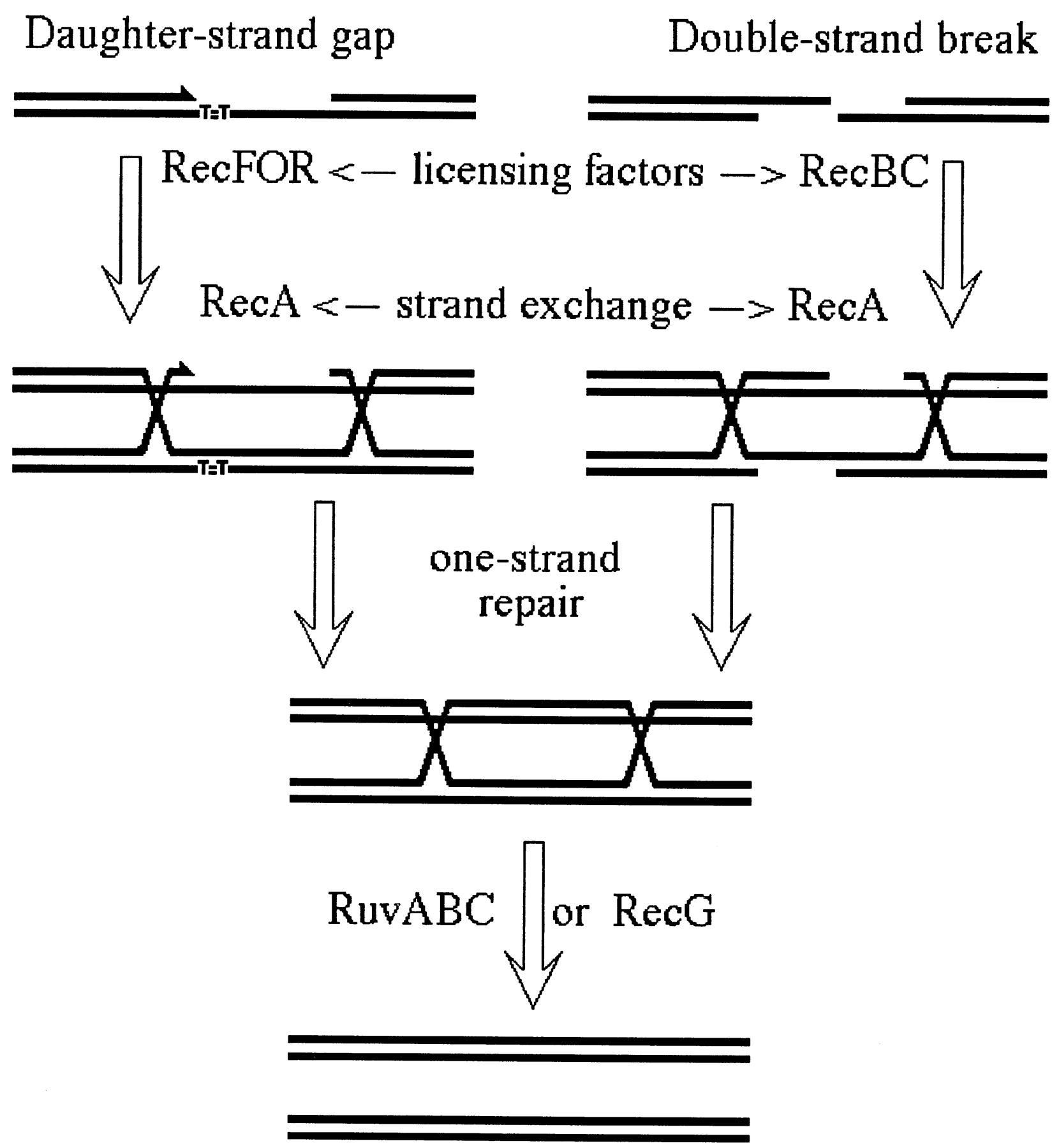
A scheme of recombinational repair in E. coli. RecFOR activities license RecA polymerization of daughter-strand gaps, whereas the RecBC enzyme does the same for double-strand breaks. After the RecA-catalyzed homologous strand exchange enables one-strand repair (excision repair) to fix the irregularities in the individual DNA strands, RuvABC or RecG activities remove the spent RecA filaments and Holliday junctions from the repair intermediate, freeing the participating chromosomes.
Explanation:
Enzymes of known biochemical activities are shown. The presynaptic steps result in the formation of a RecA filament. At gaps, this step requires RecJ, RecF, RecO, and RecR: the 5′ ssDNA exonuclease RecJ enlarges the ssDNA region (possibly with the help of various helicases, as no specific helicase is required for gap repair); RecF, RecO, and RecR promote RecA binding to SSB-coated DNA. At dsDNA ends, RecBCD (AddAB in B. subtilis) degrades DNA until it encounters a χ site; its helicase-nuclease activity is then modified to produce a 3′-ended ssDNA, to which it loads RecA. The synaptic step (homology search and strand exchange) is always performed by RecA and results in the formation of a Holliday junction (X structure). The postsynaptic steps are the migration and the resolution of Holliday junctions. Migration can be performed by RuvAB or by RecG, and resolution is made by RuvC (RecU in B. subtlis; RuvC forms a complex with RuvAB in E. coli). In addition, RecBCD-mediated recombination is always coupled with PriA-dependent replication restart. Antirecombinases are not shown: UvrD and MutLS prevent by different means the strand exchange reaction. In recBC mutants, the presynaptic steps of dsDNA end repair can be catalyzed by the helicase RecQ and the gap repair proteins RecJ and RecFOR, a reaction that is prevented by SbcB (and SbcCD) nucleases.
Answer:
crossing over
Explanation:
This occurs between prophase 1 and metaphase 1.