Answer:


Step-by-step explanation:
We know that ,
Null hypothesis is a statement that contains the population parameter to represent claim or against claim statement along with = , ≤ and ≥ signs.
is a statement that contains the population parameter to represent claim or against claim statement along with = , ≤ and ≥ signs.
Where as Alternative hypothesis is also a statement that contains the population parameter but against the null hypothesis . It contains ≠ , < and > signs.
is also a statement that contains the population parameter but against the null hypothesis . It contains ≠ , < and > signs.
In the given situation, the population parameter is population mean  .
.
Claim: The mean pulse rate (in beats per minute, or bpm) of adult males is equal to 69 bpm.
i.e. 
and  [Against
[Against 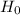 ]
]
Answer:
478miles
Step-by-step explanation:
32=1gallons 32×15 = 510miles
15g
510- 32= 478miles
The first step would be to plug in the numbers and see if the equation is true
Count be ten for the y axis
Answer:
I-
Step-by-step explanation:
) 5)8%2=80)4)<em>88</em><em>=</em><em>5</em><em>=</em><em>5</em><em>=</em><em>88</em><em>=</em><em><u>9</u></em><em><u>=</u></em><em><u>5</u></em><em><u>)</u></em><em><u>5</u></em><em><u>=</u></em>