The correct answer for this question is 'False'
Environmental science is not the study of the earth's natural hazards
What is Environmental science?
Environmental Science is the study of physical, chemical, biological components of earth's natural environment they include air, water etc.,
The Environmental science closely studies the human impact on the earth's environment.
This field of science also helps us to know about how science effects our environment.
And the 3 main aspects of Environmental science are:
To understand the interaction between humans and environment
To understand how natural world works
To Find a way to deal with the environmental problems to live sustainably.
To know more about environmental science, click here:
brainly.com/question/13254058
#SPJ4
U-238
The number besides the U means its total molar mass. The molar mass of this element is 238
Molar mass= protons + neutrons
This means that 238= 146 + protons
Do 238 - 146
Answer is 92
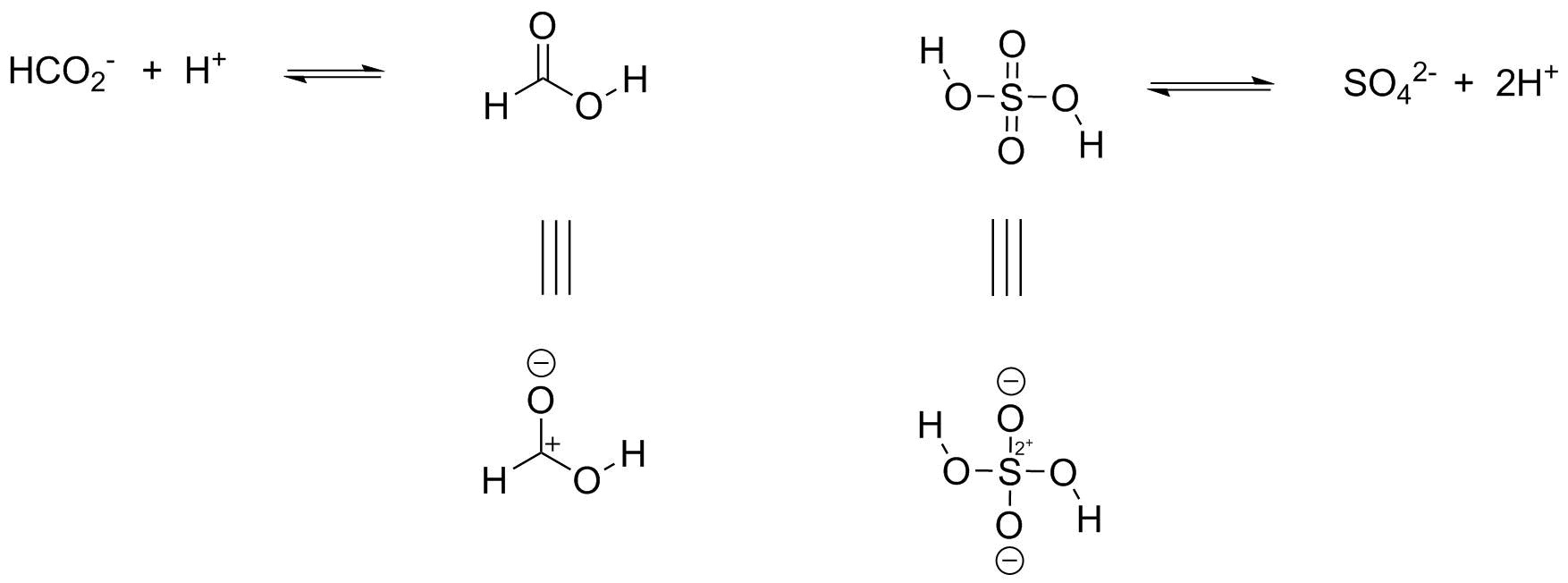
Among formic acid (HCOOH ) and sulfuric acid (H₂SO₄), formic acid is the weak acid. Acidic strength of any acid is the tendency of that acid to loose proton. Among these two acids formic acid has a pKa value of 3.74 greater than that of sulfuric acid i.e. -10. Remember! Greater the pKa value of acid weaker is that acid and vice versa. Below I have drawn the Ionization of both acids to corresponding conjugate bases and protons. The structures below with charges are drawn in order to explain the reason for strength. As it is seen in charged structure of formic acid, there is one positive charge on carbon next to oxygen carrying proton. The electron density is shifted toward carbon as it is electron deficient and demands more electron hence, attracting electron density from oxygen and making the oxygen hydrogen bond more polar. While, in case of sulfuric acid it is depicted that Sulfur attached to oxygen containing proton has 2+ charge, means more electron deficient as compared to carbon of formic acid, hence, more electron demanding and strongly attracting electrons from oxygen and making the oxygen hydrogen bond very polar and highly ionizable.
Answer:
Explanation:
Taking into account the definition of Avogadro's Number, the correct answers are:
1 mole of any element contains 6.023×10²³ atoms
1 mole of any compound contains 6.023×10²³ molecules
You have to know that Avogadro's Number or Avogadro's Constant is called the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance. Its value is 6.023×10²³ particles per mole.
Avogadro's number represents a quantity without an associated physical dimension, so it is considered a pure number that allows describing a physical characteristic without an explicit dimension or unit of expression.
Avogadro's number applies to any substance, because the number of elementary units in a mole of a substance is, by definition, a constant that does not depend on the material or the type of particle considered.
So, in this case, the correct answers are:
1 mole of any element contains 6.023×10²³ atoms
1 mole of any compound contains 6.023×10²³ molecules
hope that help you mark me as brinilylist pls
The SI base units and their physical quantities are the metre for measurement of length, the kilogram for mass, the second for time, the ampere for electric current, the kelvin for temperature, the candela for luminous intensity, and the mole for amount of substance.