First of all, you must understand that the average life span of a person is from 70 yrs. throughout the world. See (Picture 1). Life expectancy is measured by the average of each individual who have lived inside a nation or area. It then takes into account how long they live. Was there a ongoing war affecting that area? Was there pestilence, famine, or any natural disasters? These are some examples that would affect the population of a certain demographic, and can lead to people living longer or shorter, which in turn can fluctuate the total amount in the world population. In the case of a longevity of the race of humanity, there are many things to look at.
Firstly, the ever expanding usage of the ground to furbish the energy needed for the sustainability of life. One of the many necessities that a person must meet is having access to a stable source of food, as well as water. The first part comes in the form of gathering, or finding edible food while staying away from those that are harmful. This typically came in a trial-and-error format, and gave those who survived a broader knowledge of what can be eaten, and what cannot be. The second part is the advancement of tools such as spears using flint rock, or forging of sharp wooden staves that can be used to hunt game or fish. Furbishing of meat into the diet led to people generally having more muscle strength, as well as as well as retaining muscle mass, building stronger bones and absorbing iron much more better, which is essential in blood production. Whenever there is a large and stable source of food, as well as later having a globalization trading effort, the world population would grow. Stability almost always lead to higher population growth.
Secondly, the practition of medicine and advancement in the medical field. Medicine plays an important role in not only stabilizing the homeostasis of the body, but also to prescribe a positive role in preparing the human body to develop anti-bodies to counter any new diseases (to the individual) by giving doses of weakened traits. The usage of medicine and other ways in keeping a person healthy (such as sewage systems to drain away wastes full of germs), and the advancement of medicine to negate new diseases have allowed people to survive any incidents and live longer.
Thirdly, the stability of the world (as in wars). Having smaller wars, or little to no war can affect the stability of an economic, political, and social climate of a country. The more balanced and secure it is, the more a group is willing to have children in most cases. However, that is not too say that this means that the growth rate is increasing. Stability also brings, as studies would typically show, a typical mindset for people to make more money in their early adulthood in which case to save for the future. This means putting aside finding a family, or even having kids. Marrying later typically means that the usual ages of childbearing and rearing are on it's waning stages or have already passed, in which it means that the growth is slacking. However, remember the point that I brought up that people are living longer now. This means that the world population is expanding, but not because of new population, but because of the longevity of the old. (See Graph 2).
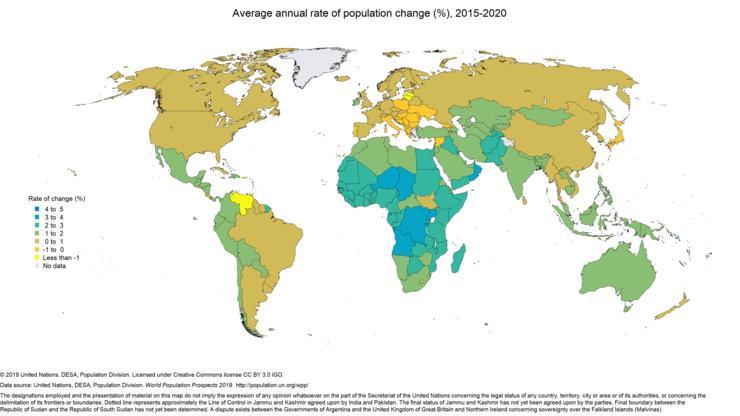
Finally, you must understand that growth rate as a whole is not as low as expected. The growth rate can be different from country to country. Countries that typically lean towards 2nd or 3rd world countries typically still have a larger growth rate. These generally account for the large amount of world population, even though as a general rule the growth rate is decreasing in most developed countries. Take a look at the graphs shown below (picture 3 & 4). The 3rd one shows the GDP as comparable to each individual countries, which in turn typically means if the country falls under which world category.
In the end, the World Population is made up of many different individual countries, in which each experience it's own growths or set backs in population sizes. One must also take into account the movement of people through countries (typically from developing to developed) in sustaining of other places. Also, one must take into account that not only stability, but also necessity would influence a persons decision to have children, which in turn would affect the growth rate. Advancement in providing the basic necessities and health services to a person would raise the longevity, which means that while not only the population is increasing, but that the population is leaning more towards the older side then younger. Finally, the world population has a peak in limit, and that once the limit is hit, the growth rate and roof would cave the population and lower it down, typically through diseases, famine, or other natural disasters.