1. When our Galaxy was forming why was the center dense?
Answer: <u>Stars are believed to originate as from gas clouds in space. Gravitational forces cause this gas to contract forming a dense center of gravitational force.Eventually this extreme pressure at the center of the star causes nuclear fusion reactions to occur.</u>
2. Why did hydrogen atoms collide in the dense center of the Galaxy?
Answer: <u>Compression would heat the gas to temperatures above 1,000 kelvins. Some hydrogen atoms would pair up in the dense, hot gas, creating trace amounts of molecular hydrogen. The hydrogen molecules would then start to cool the densest parts of the gas by emitting infrared radiation after they collide with hydrogen atoms.</u>
The Aufbau principle states that, hypothetically, electrons orbiting one or more atoms fill the lowest available energy levels before filling higher levels (e.g., 1s before 2s). In this way, the electrons of an atom, molecule, or ion harmonize into the most stable electron configuration possible.
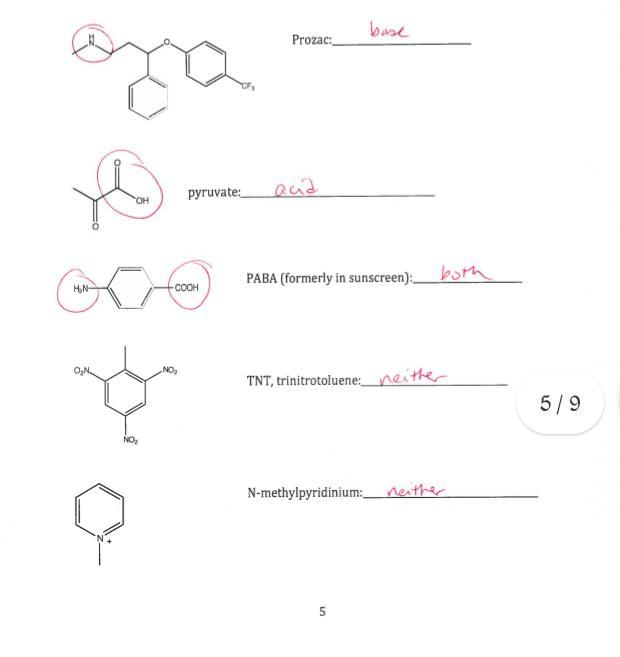
Answer:
Both
Explanation:
produce OH− (hydroxide) ions. According to this view, an acid–base reaction involves the reaction of a proton with a hydroxide ion to form water. Although Brønsted and Lowry defined an acid similarly to Arrhenius by describing an acid as any substance that can donate a proton, the Brønsted–Lowry definition of a base is much more general than the Arrhenius definition. In Brønsted–Lowry terms, a base is any substance that can accept a proton, so a base is not limited to just a hydroxide ion. This means that for every Brønsted–Lowry acid, there exists a corresponding conjugate base with one fewer proton, as we demonstrated in Chapter 4 "Reactions in Aqueous Solution". Consequently, all Brønsted–Lowry acid–base reactions actually involve two conjugate acid–base pairs and the transfer of a proton from one substance (the acid) to another (the base). In contrast, the Lewis definition of acids and bases, discussed in Chapter 8 "Ionic versus Covalent Bonding", focuses on accepting or donating pairs of electrons rather than protons. A Lewis base is an electron-pair donor, and a Lewis acid is an electron-pair acceptor.
Answer:
1.667L of a 0.30M BaCl₂ solution
Explanation:
<em>Of a 0.30M barium chloride, contains 500.0mmol of barium chloride.</em>
<em />
Molarity is an unit of concentration used in chemistry defined as the moles of solute present in 1 liter of solution.
In a 0.30M BaCl₂ solution there are 0.30 moles of BaCl₂ in 1 liter of solution.
Now, in your solution you have 500mmol of BaCl₂ = 0.500 moles of BaCl₂ (1000 mmol = 1 mol). Thus, 0.500 moles of BaCl₂ are present in:
0.500 moles * (1L / 0.30 moles) =
<h3>1.667L of a 0.30M BaCl₂ solution</h3>
Answer: Here's your answer: Therefore, the decimal number 0.0020 written in scientific notation is 2 × 10-3 and it has 2 significant figures. Here are some more examples of decimal to scientific notation 0.00200 in scientific notation
Explanation: Pls mark me brainiest pls