STP is abbreviation for Standard Temperature and Pressure at which the temperature is 273 K and pressure is 1 atm
- At these conditions the molar volume is equal to 22.4 L
so 1 mole of SO₂ volume = 22.4 L
? mole of SO₂ volume = 2.5 L
number of moles = 2.5 / 22.4 = 0.1116 mol
mass of SO₂ = 0.1116 * 64.063 = 7.15 g
Answer:
Both diseases affect the control of voluntary muscles.
Explanation:
Parkinson's disease is a progressive brain disease that affects movement. It affects the nerve cells that produce dopamine in the part of the brain called substantia nigra. The symptoms include shaking, stiffness, and difficulty with walking, balance, and coordination. Symptoms get worse with time, often leaving people with difficulty walking and talking.
ALS (amyotrophic lateral sclerosis) is a progressive nervous system disease that affects nerve cells in the brain and spinal cord. The first symptoms usually involve muscle weakness, and as the disease progresses, it results in the loss of muscle control.
Scientists don't know the exact cause of these diseases. As the cause is not known, there is no exact way to prevent them. There is no cure for them, either. The treatment is focused on the management of symptoms.
This is why the third option is the correct one.
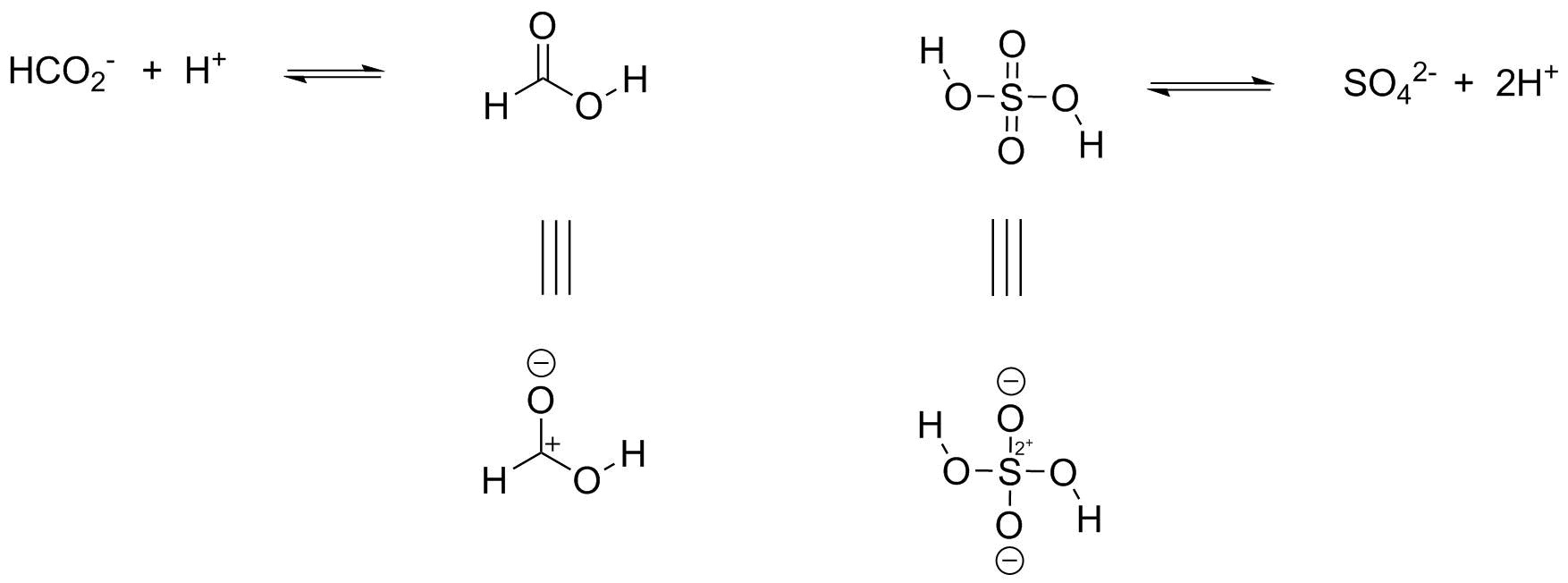
Among formic acid (HCOOH ) and sulfuric acid (H₂SO₄), formic acid is the weak acid. Acidic strength of any acid is the tendency of that acid to loose proton. Among these two acids formic acid has a pKa value of 3.74 greater than that of sulfuric acid i.e. -10. Remember! Greater the pKa value of acid weaker is that acid and vice versa. Below I have drawn the Ionization of both acids to corresponding conjugate bases and protons. The structures below with charges are drawn in order to explain the reason for strength. As it is seen in charged structure of formic acid, there is one positive charge on carbon next to oxygen carrying proton. The electron density is shifted toward carbon as it is electron deficient and demands more electron hence, attracting electron density from oxygen and making the oxygen hydrogen bond more polar. While, in case of sulfuric acid it is depicted that Sulfur attached to oxygen containing proton has 2+ charge, means more electron deficient as compared to carbon of formic acid, hence, more electron demanding and strongly attracting electrons from oxygen and making the oxygen hydrogen bond very polar and highly ionizable.
The ammonia gas is absorbed in the concentrated brine to produce aqueous sodium chloride and aqueous ammonia. This ammoniation process is exothermic, so energy is released as heat. The ammonia tower eventually needs to be cooled.
HCl :
hydrochloric acid
answer A
hope this helps!