Answer:
Δ = 26.20 kJ/mol
= 26.20 kJ/mol
Lattice Energy ( - Δ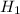 ) = - 656.20 kJ/mol
) = - 656.20 kJ/mol
Explanation:
Given that:
the mass of weight of solute present = 1.81 g
number of moles of NH₄NO₃ = 
number of moles of NH₄NO₃ = 
number of moles of NH₄NO₃ = 0.023 mol
The equation for the dissolution of NH₄NO₃ can be written as:

Since heat is absorbed by the salt ( NH₄NO₃) after dissolution of the salt in the water: we have;
Heat absorbed by the NH₄NO₃ = Heat evolved by the solution.
Let first determine the heat evolved by the solution in order to find the amount of heat absorbed by the NH₄NO₃.
Heat of solution is given as:

where m = total mass of the weight of the solution = 1.81 g + 85.00 g = 86.81 g
 = 25.00°C - 23.34°C
= 25.00°C - 23.34°C
 = 1.66°C
= 1.66°C
S = specific heat capacity of the solution which is given as : 4.18 J/g °C
 (since heat is lost by the water to the compound)
(since heat is lost by the water to the compound)
= -86.81 g × 4.18 J/g °C × 1.66°C
= - 602.357228 J
= - 602.36 J
This same amount of heat is absorbed by 1.81 g of NH₄NO₃ (0.023 mol)
Hence; The amount of heat absorbed by 1 mole of NH₄NO₃
= 
= 
= 26189.565 × 0.001
= 26.189565
≅ 26.20 kJ/mol
Hence, the Δ = 26.20 kJ/mol
= 26.20 kJ/mol
b)
The heat of solution Δ = Δ
= Δ + Δ
+ Δ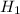
where;
Δ = the enthalpy of hydration for NH₄NO₃ is -630. kJ/mol
= the enthalpy of hydration for NH₄NO₃ is -630. kJ/mol
Δ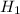 is said to be the energy needed for dissociation of the NH₄NO₃ in the solution.
is said to be the energy needed for dissociation of the NH₄NO₃ in the solution.
∴ from the above equation;
Δ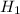 = Δ
= Δ - Δ
- Δ
Δ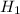 = 26.20 kJ/mol - (- 630 kJ/mol)
= 26.20 kJ/mol - (- 630 kJ/mol)
Δ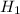 = 26.20 kJ/mol + 630 kJ/mol
= 26.20 kJ/mol + 630 kJ/mol
Δ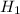 = 656.20 kJ/mol
= 656.20 kJ/mol
However, Lattice Energy = - Δ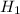
∴ Lattice Energy = - 656.20 kJ/mol