Answer:
Initial temperature = 170. 414 °C
Total mass = 94.478 Kg
Final volumen = 33.1181 m^3
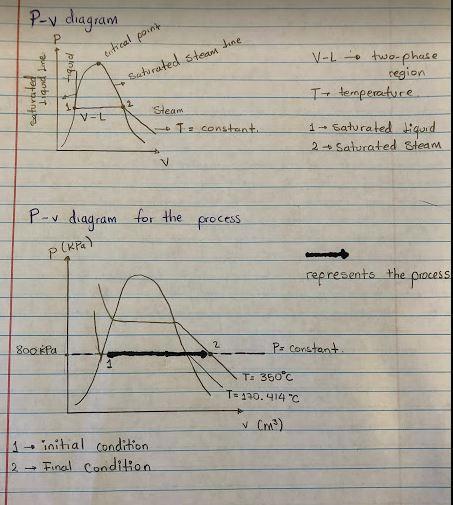
Diagram = see picture.
Explanation:
We can consider this system as a close system, because there is not information about any output or input of water, so the mass in the system is constant.
The information tells us that the system is in equilibrium with two phases: liquid and steam. When a system is a two phases region (equilibrium) the temperature and pressure keep constant until the change is completed (either condensation or evaporation). Since we know that we are in a two-phase region and we know the pressure of the system, we can check the thermodynamics tables to know the temperature, because there is a unique temperature in which with this pressure (800 kPa) the system can be in two-phases region (reach the equilibrium condition).
For water in equilibrium at 800 kPa the temperature of saturation is 170.414 °C which is the initial temperature of the system.
to calculate the total mass of the system, we need to estimate the mass of steam and liquid water and add them. To get these values we use the specific volume for both, liquid and steam for the initial condition. We can get them from the thermodynamics tables.
For the condition of 800 kPa and 170.414 °C using the thermodynamics tables we get:
Vg (Specific Volume of Saturated Steam) = 0.240328 m^3/kg
Vf (Specific Volume of Saturated Liquid) = 0.00111479 m^3/kg
if you divide the volume of liquid and steam provided in the statement by the specific volume of saturated liquid and steam, we can obtain the value of mass of vapor and liquid in the system.
Steam mass = *0.9 m^3 / 0.240328 m^3/kg = 3.74488 Kg
Liquid mass = 0.1 m^3 /0.00111479 m^3/kg = 89.70299 Kg
Total mass of the system = 3.74488 Kg + 89.70299 Kg = 93,4478 Kg
If we keep the pressure constant increasing the temperature the system will experience a phase-change (see the diagram) going from two-phase region to superheated steam. When we check for properties for the condition of P= 800 kPa and T= 350°C we see that is in the region of superheated steam, so we don’t have liquid water in this condition.
If we want to get the final volume of the water (steam) in the system, we need to get the specific volume for this condition from the thermodynamics tables.
Specific Volume of Superheated Steam at 800 kPa and 350°C = 0.354411 m^3/kg
We already know that this a close system so the mass in it keeps constant during the process.
If we multiply the mass of the system by the specific volume in the final condition, we can get the final volume for the system.
Final volume = 93.4478 Kg * 0.354411 m^3/kg = 33.1189 m^3
You can the P-v diagram for this system in the picture.
For the initial condition you can calculate the quality of the steam (measure of the proportion of steam on the mixture) to see how far the point is from for the condition on all the mix is steam. Is a value between 0 and 1, where 0 is saturated liquid and 1 is saturated steam.
Quality of steam = mass of steam / total mass of the system
Quality of steam = 3.74488 Kg /93.4478 Kg = 0,040 this value is usually present as a percentage so is 4%.
Since this a low value we can say that we are very close the saturated liquid point in the diagram.