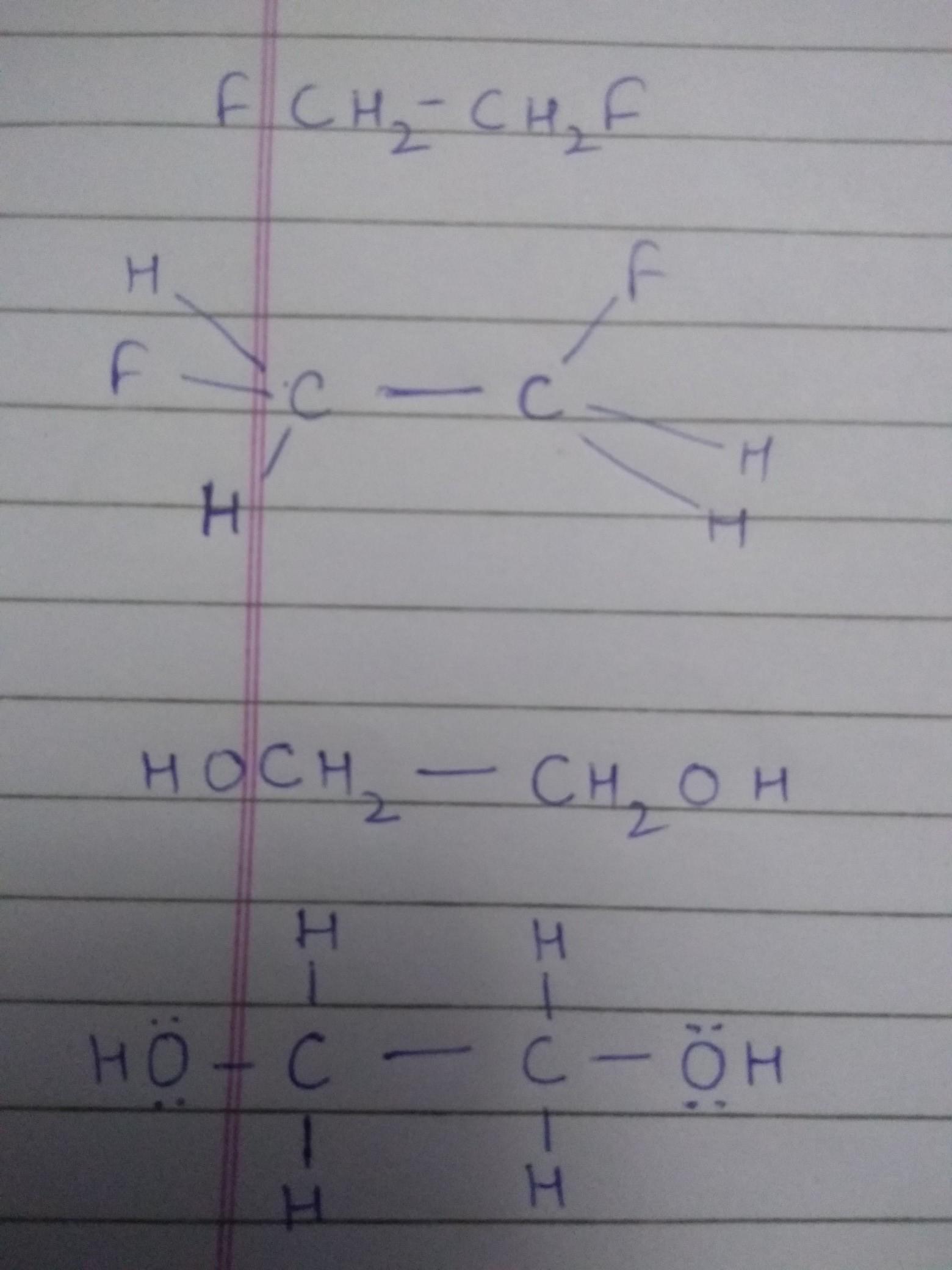
Ans-A
<span>In CaF2, the oxidation number of Ca is +2,& that of F is -1.
Ans-B
</span><span>In H2SO4, the oxidation number of H is +1, that of S is +6,& that of O is -2.
</span>
Ans-C
<span>In CaSO4, the oxidation number of Ca is +2, that of S is +6,& that of O is -2.
Ans-D
</span><span>In HF, the oxidation number of H is +1,& that of F is -1.
</span>
Explanation:
<span>Oxidation number is a number that is assigned to an element in a compound, which shows the number of electrons gained or lost by an atom.</span>
Rules:
1. If the element is ALONE in the chemical equation, and it is NOT an ION, its oxidation number will ALWAYS be zero.<span>
2. When there is an ION in the equation, its oxidation number will ALWAYS be its ionic number.</span><span>
A(2 parts):
</span>The oxidation number of Ca & F?<span>
In what group Ca lies? Well in group 2 in the periodic table.
Is it alone? No, it is not. It's with Florine F.
Is it multiple of itself? No.
The second element is Florine(F).
</span>In what group F lies? Well in group 17(in halogens) in the periodic table.
Is it multiple of itself? Yes. There are 2 Florine atoms.
<span>
Is there any net charge on the compound? No.</span>
Blank 2: The oxidation number of F2 = 2 * (-1) = -2. Since the oxidation number of the single atom F is -1 for the halogens(group-17 members). In this compound there are 2 Florine atoms, therefore it should be -2. Although the F2 has the oxidation number of -2, the single atom of F has the oxidation number -1.
Blank 1: The oxidation number of Calcium= +2. Since the oxidation number of the single atom Ca is +2 for the group-2 members. Also, we can recheck it by using the Rule-2, mentioned above, but for compound. As I mentioned before, the net charge on the CaF2 is zero; therefore, the sum of the oxidation number of Ca and that of F2 has to be zero. Since the oxidation number of F2 is -2, the oxidation number of Ca has to be +2 to make the net charge equals to zero. Therefore, the oxidation number of Ca is +2.
B(3 parts):
<span>The oxidation number of H, S & O?
</span>
In what group Hydrogen(H) lies? Well in group 1 in the periodic table.
Is it alone? No, it is not. It's with Sulfur(S) and Oxygen(O) .
Is it multiple of itself? Yes. It's H2; therefore, there are two hydrogen atoms.
The second element is Sulfur(S).
In what group S lies? Well in group 16(in chalcogens) in the periodic table.
Is it multiple of itself? No.
The third element is Oxygen(O).
In what group Oxygen(O) lies? Well in group-16 in the periodic table.
Is it alone? No, it is not. It's with Sulfur(S) and Hydrogen(H) .
Is it multiple of itself? Yes. It's O4; therefore, there are four oxygen atoms.
Is there any net charge on the compound? No.
Blank 3: The oxidation number of O4 = 4 * (-2) = -8. Since the oxidation number of the single atom O is -2 for the chalcogens (group-16 members). In this compound there are 4 Oxygen atoms, therefore it should be -8. Although the O4 has the oxidation number of -8, the single atom of O has the oxidation number -2.
Blank 1: The oxidation number of H2 = 2 * (+1) = +2. Since the oxidation number of the single atom H is +1 for the group-1 members. In this compound there are 2 hydrogen atoms, therefore it should be +2. Although the H2 has the oxidation number of +2, the single atom of H has the oxidation number +1.
Blank 2: As I mentioned before, the net charge on the H2SO4 is zero; therefore, the sum of the oxidation number of H2, S and that of O4 has to be zero. Since the oxidation number of H2 is +2, and the oxidation number of O4 is -8, the oxidation number of Sulfur has to be +6 to make the net charge equals to zero. Therefore, the oxidation number of S is +6.
C(3 parts):
Blank 3: The oxidation number of O4 = 4 * (-2) = -8. Since the oxidation number of the single atom O is -2 for the chalcogens (group-16 members). In this compound there are 4 Oxygen atoms, therefore it should be -8. Although the O4 has the oxidation number of -8, the single atom of O has the oxidation number -2.
Blank 1: The oxidation number of Calcium= +2. Since the oxidation number of the single atom Ca is +2 for the group-2 members. Although Sulfur in the compound is also a single element, but as Calcium comes first, therefore, we would consider Ca as an independent element. Hence, Ca has the oxidation number +2.
Blank 2: As I mentioned before, the net charge on the CaSO4 is zero; therefore, the sum of the oxidation number of Ca, S and that of O4 has to be zero. Since the oxidation number of Ca is +2, and the oxidation number of O4 is -8, the oxidation number of Sulfur has to be +6 to make the net charge equals to zero. Therefore, the oxidation number of S is +6.
D(2 parts):
Blank 2: The oxidation number of F = -1. Since the oxidation number of the single atom F is -1 for the halogens(group-17 members).
Blank 1: The oxidation number of Hydrogen H = +1. As I mentioned before, the net charge on the HF is zero; therefore, the sum of the oxidation number of H and that of F has to be zero. Since the oxidation number of F is -1, the oxidation number of H has to be +1 to make the net charge equals to zero. Therefore, the oxidation number of H is +1.
<span>In CaF2, the oxidation number of Ca is +2,& that of F is -1.
Ans-B
</span><span>In H2SO4, the oxidation number of H is +1, that of S is +6,& that of O is -2.
</span>
Ans-C
<span>In CaSO4, the oxidation number of Ca is +2, that of S is +6,& that of O is -2.
Ans-D
</span><span>In HF, the oxidation number of H is +1,& that of F is -1.
</span>
Explanation:
<span>Oxidation number is a number that is assigned to an element in a compound, which shows the number of electrons gained or lost by an atom.</span>
Rules:
1. If the element is ALONE in the chemical equation, and it is NOT an ION, its oxidation number will ALWAYS be zero.<span>
2. When there is an ION in the equation, its oxidation number will ALWAYS be its ionic number.</span><span>
A(2 parts):
</span>The oxidation number of Ca & F?<span>
In what group Ca lies? Well in group 2 in the periodic table.
Is it alone? No, it is not. It's with Florine F.
Is it multiple of itself? No.
The second element is Florine(F).
</span>In what group F lies? Well in group 17(in halogens) in the periodic table.
Is it multiple of itself? Yes. There are 2 Florine atoms.
<span>
Is there any net charge on the compound? No.</span>
Blank 2: The oxidation number of F2 = 2 * (-1) = -2. Since the oxidation number of the single atom F is -1 for the halogens(group-17 members). In this compound there are 2 Florine atoms, therefore it should be -2. Although the F2 has the oxidation number of -2, the single atom of F has the oxidation number -1.
Blank 1: The oxidation number of Calcium= +2. Since the oxidation number of the single atom Ca is +2 for the group-2 members. Also, we can recheck it by using the Rule-2, mentioned above, but for compound. As I mentioned before, the net charge on the CaF2 is zero; therefore, the sum of the oxidation number of Ca and that of F2 has to be zero. Since the oxidation number of F2 is -2, the oxidation number of Ca has to be +2 to make the net charge equals to zero. Therefore, the oxidation number of Ca is +2.
B(3 parts):
<span>The oxidation number of H, S & O?
</span>
In what group Hydrogen(H) lies? Well in group 1 in the periodic table.
Is it alone? No, it is not. It's with Sulfur(S) and Oxygen(O) .
Is it multiple of itself? Yes. It's H2; therefore, there are two hydrogen atoms.
The second element is Sulfur(S).
In what group S lies? Well in group 16(in chalcogens) in the periodic table.
Is it multiple of itself? No.
The third element is Oxygen(O).
In what group Oxygen(O) lies? Well in group-16 in the periodic table.
Is it alone? No, it is not. It's with Sulfur(S) and Hydrogen(H) .
Is it multiple of itself? Yes. It's O4; therefore, there are four oxygen atoms.
Is there any net charge on the compound? No.
Blank 3: The oxidation number of O4 = 4 * (-2) = -8. Since the oxidation number of the single atom O is -2 for the chalcogens (group-16 members). In this compound there are 4 Oxygen atoms, therefore it should be -8. Although the O4 has the oxidation number of -8, the single atom of O has the oxidation number -2.
Blank 1: The oxidation number of H2 = 2 * (+1) = +2. Since the oxidation number of the single atom H is +1 for the group-1 members. In this compound there are 2 hydrogen atoms, therefore it should be +2. Although the H2 has the oxidation number of +2, the single atom of H has the oxidation number +1.
Blank 2: As I mentioned before, the net charge on the H2SO4 is zero; therefore, the sum of the oxidation number of H2, S and that of O4 has to be zero. Since the oxidation number of H2 is +2, and the oxidation number of O4 is -8, the oxidation number of Sulfur has to be +6 to make the net charge equals to zero. Therefore, the oxidation number of S is +6.
C(3 parts):
Blank 3: The oxidation number of O4 = 4 * (-2) = -8. Since the oxidation number of the single atom O is -2 for the chalcogens (group-16 members). In this compound there are 4 Oxygen atoms, therefore it should be -8. Although the O4 has the oxidation number of -8, the single atom of O has the oxidation number -2.
Blank 1: The oxidation number of Calcium= +2. Since the oxidation number of the single atom Ca is +2 for the group-2 members. Although Sulfur in the compound is also a single element, but as Calcium comes first, therefore, we would consider Ca as an independent element. Hence, Ca has the oxidation number +2.
Blank 2: As I mentioned before, the net charge on the CaSO4 is zero; therefore, the sum of the oxidation number of Ca, S and that of O4 has to be zero. Since the oxidation number of Ca is +2, and the oxidation number of O4 is -8, the oxidation number of Sulfur has to be +6 to make the net charge equals to zero. Therefore, the oxidation number of S is +6.
D(2 parts):
Blank 2: The oxidation number of F = -1. Since the oxidation number of the single atom F is -1 for the halogens(group-17 members).
Blank 1: The oxidation number of Hydrogen H = +1. As I mentioned before, the net charge on the HF is zero; therefore, the sum of the oxidation number of H and that of F has to be zero. Since the oxidation number of F is -1, the oxidation number of H has to be +1 to make the net charge equals to zero. Therefore, the oxidation number of H is +1.
5
0