Answer:
5 ) The mass, 6) with lubrication and using surfaces that are not rough
Explanation:
5) If two bodies are held regardless of their densities and can be combined by some chemical or physical process, the only physical property to be modified will be the mass of the resulting body.
8)
Friction depends on the contact between two surfaces and when a body has a relative motion with respect to a contact surface. In order to reduce friction the contact surface must be lubricated, also the friction depends on the coefficient of friction between surfaces and the normal force exerted by the surface parallel to the area of contact with the body. Mathematically it can be expressed with the following equation.
![F_{f} = u*N\\where:\\u = friction coefficient\\N = normal force [Newtons]\\F_{f}= friction force [Newtons]](https://tex.z-dn.net/?f=F_%7Bf%7D%20%3D%20u%2AN%5C%5Cwhere%3A%5C%5Cu%20%3D%20friction%20coefficient%5C%5CN%20%3D%20normal%20force%20%5BNewtons%5D%5C%5CF_%7Bf%7D%3D%20friction%20force%20%5BNewtons%5D)
Answer:
D. The cart is moving at a constant speed or velocity
Explanation:
Equilibrium is a state of body in which it is either at rest or moves with uniform velocity. The sum of forces acting on such a body is always zero and the sum of all the torques acting on it is also zero.
There are two types of equilibrium as follows:
Static Equilibrium: When a body is at rest it is said to be in static equilibrium.
Dynamic Equilibrium: When a body is moving with constant velocity, then it is said to be in dynamic equilibrium.
Hence, the correct option here will be:
<u>D. The cart is moving at a constant speed or velocity</u>
Answer:
a. the time required for the onset of evaporation is: 196.1 seconds and b. the time required for all of the water to evaporate is: 1328.3 seconds.
Explanation:
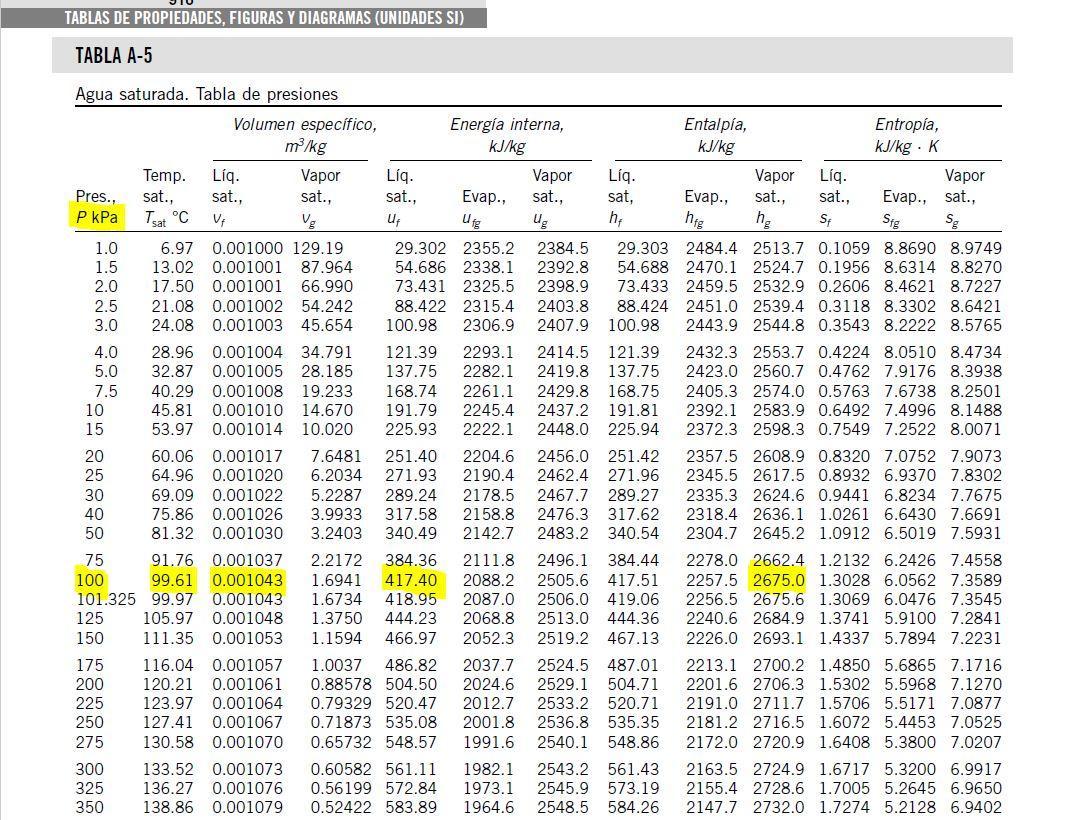
We need to stablish that there is 3 states at this problem. At the firts one, water is compressed liquid and the conditions for this state are: P1=100KPa,T1=20°C,V1=0.5m^3. From the compressed liquid chart and using extrapolation, we can get: v1=vf1=0.0010018 (m^3/Kg) and u1=uf1=83.95(KJ/kg). Now we can find the mass of water at the state 1 as:  Then the liquid water is heated at a rate of 0.85KW, and its volume increase, while work is done by the system at the boundary, we can assume that the pressure remains constant throughout the entire process. At the second state the water is saturated liquid and the conditions are: P2=100KPa, T2=Tsat=99.63°C, v2=vf2=0.001043(m^3/Kg) and u2=uf2=417.36(KJ/Kg). Now we can find the work as:
Then the liquid water is heated at a rate of 0.85KW, and its volume increase, while work is done by the system at the boundary, we can assume that the pressure remains constant throughout the entire process. At the second state the water is saturated liquid and the conditions are: P2=100KPa, T2=Tsat=99.63°C, v2=vf2=0.001043(m^3/Kg) and u2=uf2=417.36(KJ/Kg). Now we can find the work as: . (a) After that we need to do an energy balance for process 1-2 and get: U=Q-W or
. (a) After that we need to do an energy balance for process 1-2 and get: U=Q-W or  , solving for t we get the time required for the onset of evaporation:
, solving for t we get the time required for the onset of evaporation: .(b) Then continue heat transfer to the cooking pot and results in phase change getting vapor at 99.63°C. At the final state or third state the mass is zero because all liquid was evaporated and the initial mass at this state is the same for the second state: 0.5 (Kg) and doing an energy balances results in:
.(b) Then continue heat transfer to the cooking pot and results in phase change getting vapor at 99.63°C. At the final state or third state the mass is zero because all liquid was evaporated and the initial mass at this state is the same for the second state: 0.5 (Kg) and doing an energy balances results in: , but m3=0, now solving for t we can get the time required for all of the water to evaporate as:
, but m3=0, now solving for t we can get the time required for all of the water to evaporate as: . We can get from the saturated liquid chart the enthalpy he=hge=2675.5(KJ/Kg) @P=100KPa. Now we need to calculate the work related with the volume decreases as vapor exits the control volume or process 2-3 work boundary as:
. We can get from the saturated liquid chart the enthalpy he=hge=2675.5(KJ/Kg) @P=100KPa. Now we need to calculate the work related with the volume decreases as vapor exits the control volume or process 2-3 work boundary as:  . Now replacing every value in the time equation we get:
. Now replacing every value in the time equation we get:
Answer:
velocity of the car is 7.3516 m/s
Explanation:
M1v1a + m2v1b = m1v2a + m2v2b
775*30 + 1475*0 = 775*v2a+1475*11.9
Let v2a be x
23250 + 0 = 775x + 17552.5
775x = 23250-17552.5
775x = 5697.5
X = 7.3516 m/s
Answer:
frogs have lungs to breath on land and gills to breath underwater that makes a frog different from each other