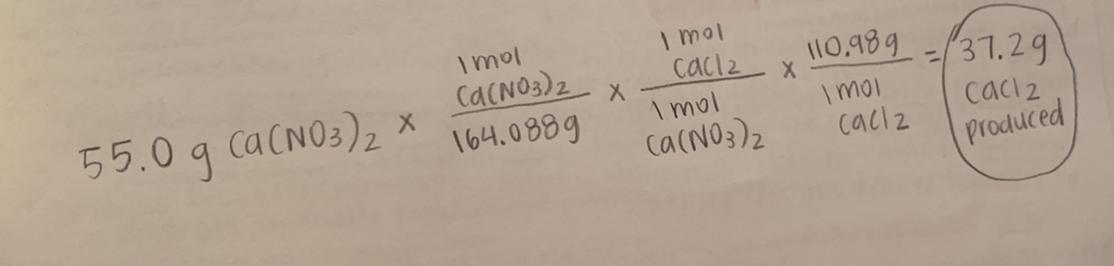Answer:
Explanation:
Cu:
Number of moles = Mass / molar masa
2 mol = mass / 64 g/mol
Mass = 128 g
Mg:
Number of moles = Mass / molar masa
0.5 mol = mass / 24 g/mol
Mass = g
Cl₂:
Number of moles = Mass / molar masa
Number of moles = 35.5 g / 24 g/mol
Number of moles = 852 mol
H₂:
Number of moles = Mass / molar mass
8 mol = Mass / 2 g/mol
Mass = 16 g
P₄:
Number of moles = Mass / molar masa
2 mol = mass / 124 g/mol
Mass = 248 g
O₃:
Number of moles = Mass / molar masa
Number of moles = 1.6 g /48 g/mol
Number of moles = 0.033 mol
H₂O
Number of moles = Mass / molar masa
Number of moles = 54 g / 18 g/mol
Number of moles = 3 mol
CO₂
Number of moles = Mass / molar masa
2 mol = mass / 124 g/mol
Mass = 248 g
NH₃
Number of moles = Mass / molar masa
Number of moles = 8.5 g / 17 g/mol
Number of moles = 0.5 mol
CaCO₃
Number of moles = Mass / molar masa
Number of moles = 100 g / 100 g/mol
Number of moles = 1 mol
a)
Given data:
Mass of iron(III)oxide needed = ?
Mass of iron produced = 100 g
Solution:
Chemical equation:
F₂O₃ + 3CO → 2Fe + 3CO₂
Number of moles of iron:
Number of moles = mass/ molar mass
Number of moles = 100 g/ 56 g/mol
Number of moles = 1.78 mol
Now we compare the moles of iron with iron oxide.
Fe : F₂O₃
2 : 1
1.78 : 1/2×1.78 = 0.89 mol
Mass of F₂O₃:
Mass = number of moles × molar mass
Mass = 0.89 mol × 159.69 g/mol
Mass = 142.124 g
100 g of iron is 1.78 moles of Fe, so 0.89 moles of F₂O₃ are needed, or 142.124 g of iron(III) oxide.
b)
Given data:
Number of moles of Al = 0.05 mol
Mass of iodine = 26 g
Limiting reactant = ?
Solution:
Chemical equation:
2Al + 3I₂ → 2AlI₃
Number of moles of iodine = 26 g/ 254 g/mol
Number of moles of iodine = 0.1 mol
Now we will compare the moles of Al and I₂ with AlI₃.
Al : AlI₃
2 : 2
0.05 : 0.05
I₂ : AlI₃
3 : 2
0.1 : 2/3×0.1 = 0.067
Number of moles of AlI₃ produced by Al are less so it will limiting reactant.
Mass of AlI₃:
Mass = number of moles × molar mass
Mass = 0.05 mol × 408 g/mol
Mass = 20.4 g
26 g of iodine is 0.1 moles. From the equation, this will react with 2 moles of Al. So the limiting reactant is Al.
c)
Given data:
Mass of lead = 6.21 g
Mass of lead oxide = 6.85 g
Equation of reaction = ?
Solution:
Chemical equation:
2Pb + O₂ → 2PbO
Number of moles of lead = mass / molar mass
Number of moles = 6.21 g/ 207 g/mol
Number of moles = 0.03 mol
Number of moles of lead oxide = mass / molar mass
Number of moles = 6.85 g/ 223 g/mol
Number of moles = 0.031 mol
Now we will compare the moles of oxygen with lead and lead oxide.
Pb : O₂
2 : 1
0.03 : 1/2×0.03 = 0.015 mol
Mass of oxygen:
Mass = number of moles × molar mass
Mass = 0.015 mol × 32 g/mol
Mass = 0.48 g
The mass of oxygen that took part in equation was 0.48 g. which is 0.015 moles of oxygen. The number of moles of Pb in 6.21 g of lead is 0.03 moles. So the balance equation is
2Pb + O₂ → 2PbO