Answer:
128.96J
Explanation:
Take g = 10m/s²
Ep = mgh
= 2.67 × 10 × 4.83 = 128.96 J
Answer:
Which of the following is the best example of a good hypothesis?
B. A cheetah can run faster than a tiger This Is A good Hypothesis Because You Can Test this With a Experiment
xXxAnimexXx
Have a great day!
Hi Spycn2115
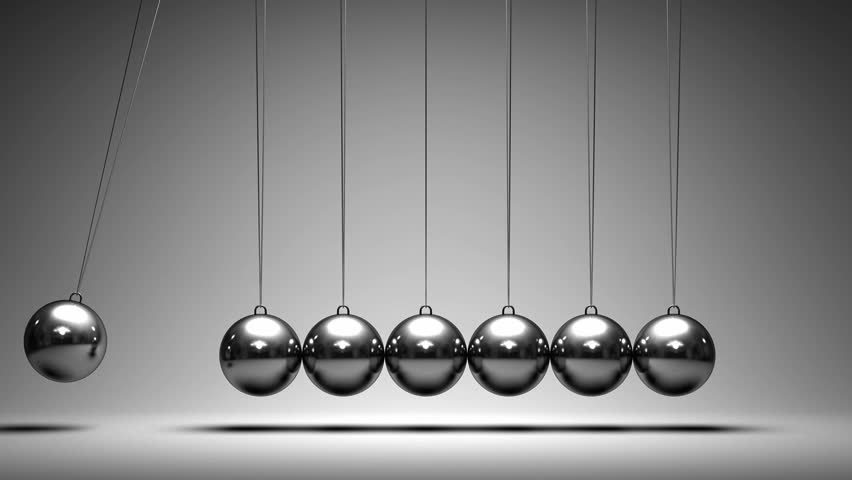
Newton's first law states that objects in motion stay in motion until an unbalanced force is acted upon it is a good fact. When I first personally heard of it in class I had no clue but when I researched it I learned that one good example of this would be those cradle balls and if u swing it, it will keep constantly hitting each other back and fourth over and over until someone grabs it and stops it, so that's what objects in motion stays in motion until an unbalanced force is acted upon it means, because the balls are in motion by moving left to right constantly and its staying in motion by not stopping and that unbalanced force is someone or something maybe hitting it or knocking it over could stop it, balanced means staying still and equal, unbalanced means unstill so moving, the cradle balls are KE and PE, (Kinect and potential energy) because kinetic means moving, so the balls are moving, and potential means stored up, so before u even swing u have the potential energy to swing it different ways before it becomes kinetic. That's one of my favorite examples of Newton's first law and I left you a pic below of cradle balls to help you get the idea.
The energy transferred by a sound wave is independent of its speed. However, the type of medium affects this due to the differences in the distances between particles in different media, i.e. sound travels faster through solids than gases as the solid particles are much closer together, so they collide more frequently allowing the sound to travel faster. Additionally, temperature affects the distances between particles in a medium, increasing them with increasing heat, causing slower sound travel. Therefore the answer is the third option.
Hope this helps!
Answer:
The resistivity of the material used to make the rod is ρ= 7.5 * 10⁻⁷ Ω.m
Explanation:
R= 0.2 Ω
L= 0.8 m
S= 1.5mm*2mm= 3 mm² = 3 * 10⁻⁶ m²
ρ = (R*S)/L
ρ= 7.5 * 10⁻⁷ Ω.m