Sorry, I can't really see the question )-:
Answer:
Together, the number of protons and the number of neutrons determine an element's mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number.
Explanation:
That number, also known as the frequency, will be larger for a short-wavelength wave than for a long-wavelength wave. The equation<span> that relates wavelength and frequency for electromagnetic waves is: λν=c where </span>λ<span> is the wavelength, ν is the frequency and c is the speed of light.</span>
Answer:
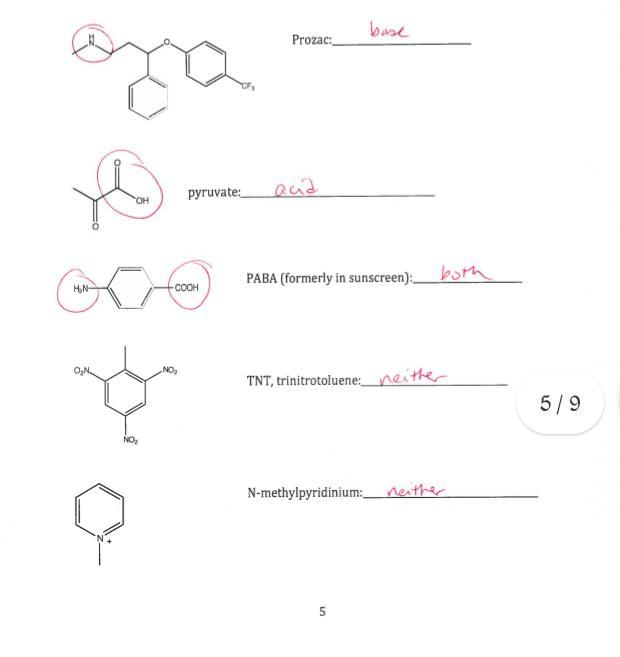
Both
Explanation:
produce OH− (hydroxide) ions. According to this view, an acid–base reaction involves the reaction of a proton with a hydroxide ion to form water. Although Brønsted and Lowry defined an acid similarly to Arrhenius by describing an acid as any substance that can donate a proton, the Brønsted–Lowry definition of a base is much more general than the Arrhenius definition. In Brønsted–Lowry terms, a base is any substance that can accept a proton, so a base is not limited to just a hydroxide ion. This means that for every Brønsted–Lowry acid, there exists a corresponding conjugate base with one fewer proton, as we demonstrated in Chapter 4 "Reactions in Aqueous Solution". Consequently, all Brønsted–Lowry acid–base reactions actually involve two conjugate acid–base pairs and the transfer of a proton from one substance (the acid) to another (the base). In contrast, the Lewis definition of acids and bases, discussed in Chapter 8 "Ionic versus Covalent Bonding", focuses on accepting or donating pairs of electrons rather than protons. A Lewis base is an electron-pair donor, and a Lewis acid is an electron-pair acceptor.
Answer:
The balloon would get larger as it gains water
Explanation:
Due to the process of osmosis, water molecules would passively move from an area of lower solute concentration, the water in the beaker, to an area of higher solute concentration, inside the balloon.