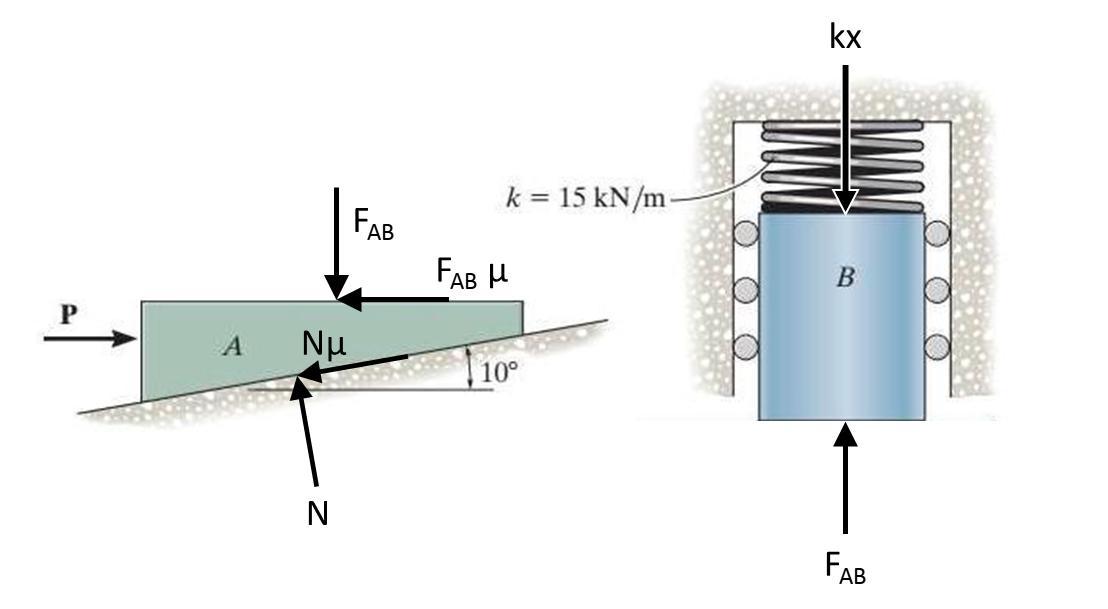
Explanation:
A) Draw free body diagrams of both blocks.
Force P is pushing right on block A, which will cause it to move right along the incline. Therefore, friction forces will oppose the motion and point to the left.
There are 5 forces acting on block A:
Applied force P pushing to the right,
Normal force N pushing up and left 10° from the vertical,
Friction force Nμ pushing down and left 10° from the horizontal,
Reaction force Fab pushing down,
and friction force Fab μ pushing left.
There are 2 forces acting on block B:
Reaction force Fab pushing up,
And elastic force kx pushing down.
(There are also horizontal forces on B, but I am ignoring them.)
Sum of forces on A in the x direction:
∑F = ma
P − N sin 10° − Nμ cos 10° − Fab μ = 0
Solve for N:
P − Fab μ = N sin 10° + Nμ cos 10°
P − Fab μ = N (sin 10° + μ cos 10°)
N = (P − Fab μ) / (sin 10° + μ cos 10°)
Sum of forces on A in the y direction:
N cos 10° − Nμ sin 10° − Fab = 0
Solve for N:
N cos 10° − Nμ sin 10° = Fab
N (cos 10° − μ sin 10°) = Fab
N = Fab / (cos 10° − μ sin 10°)
Set the expressions equal:
(P − Fab μ) / (sin 10° + μ cos 10°) = Fab / (cos 10° − μ sin 10°)
Cross multiply:
(P − Fab μ) (cos 10° − μ sin 10°) = Fab (sin 10° + μ cos 10°)
Distribute and solve for Fab:
P (cos 10° − μ sin 10°) − Fab (μ cos 10° − μ² sin 10°) = Fab (sin 10° + μ cos 10°)
P (cos 10° − μ sin 10°) = Fab (sin 10° + 2μ cos 10° − μ² sin 10°)
Fab = P (cos 10° − μ sin 10°) / (sin 10° + 2μ cos 10° − μ² sin 10°)
Sum of forces on B in the y direction:
∑F = ma
Fab − kx = 0
kx = Fab
x = Fab / k
x = P (cos 10° − μ sin 10°) / (k (sin 10° + 2μ cos 10° − μ² sin 10°))
Plug in values and solve.
x = 500 N (cos 10° − 0.4 sin 10°) / (12000 (sin 10° + 0.8 cos 10° − 0.16 sin 10°))
x = 0.0408 m
x = 4.08 cm
B) Draw free body diagrams of both blocks.
Force P is pushing block A to the right relative to the ground C, so friction force points to the left.
Block A moves right relative to block B, so friction force on A will point left. Block B moves left relative to block A, so friction force on B will point right (opposite and equal).
Block B moves up relative to the wall D, so friction force on B will point down.
There are 5 forces acting on block A:
Applied force P pushing to the right,
Normal force Fc pushing up,
Friction force Fc μ₁ pushing left,
Reaction force Fab pushing down and left 15° from the vertical,
and friction force Fab μ₂ pushing up and left 15° from the horizontal.
There are 5 forces acting on block B:
Weight force 750 n pushing down,
Normal force Fd pushing left,
Friction force Fd μ₁ pushing down,
Reaction force Fab pushing up and right 15° from the vertical,
and friction force Fab μ₂ pushing down and right 15° from the horizontal.
Sum of forces on B in the x direction:
∑F = ma
Fab μ₂ cos 15° + Fab sin 10° − Fd = 0
Fd = Fab μ₂ cos 15° + Fab sin 15°
Sum of forces on B in the y direction:
∑F = ma
-Fab μ₂ sin 15° + Fab cos 10° − 750 − Fd μ₁ = 0
Fd μ₁ = -Fab μ₂ sin 15° + Fab cos 15° − 750
Substitute:
(Fab μ₂ cos 15° + Fab sin 15°) μ₁ = -Fab μ₂ sin 15° + Fab cos 15° − 750
Fab μ₁ μ₂ cos 15° + Fab μ₁ sin 15° = -Fab μ₂ sin 15° + Fab cos 15° − 750
Fab (μ₁ μ₂ cos 15° + μ₁ sin 15° + μ₂ sin 15° − cos 15°) = -750
Fab = -750 / (μ₁ μ₂ cos 15° + μ₁ sin 15° + μ₂ sin 15° − cos 15°)
Sum of forces on A in the y direction:
∑F = ma
Fc + Fab μ₂ sin 15° − Fab cos 15° = 0
Fc = Fab cos 15° − Fab μ₂ sin 15°
Sum of forces on A in the x direction:
∑F = ma
P − Fab sin 15° − Fab μ₂ cos 15° − Fc μ₁ = 0
P = Fab sin 15° + Fab μ₂ cos 15° + Fc μ₁
Substitute:
P = Fab sin 15° + Fab μ₂ cos 15° + (Fab cos 15° − Fab μ₂ sin 15°) μ₁
P = Fab sin 15° + Fab μ₂ cos 15° + Fab μ₁ cos 15° − Fab μ₁ μ₂ sin 15°
P = Fab (sin 15° + (μ₁ + μ₂) cos 15° − μ₁ μ₂ sin 15°)
First, find Fab using the given values.
Fab = -750 / (0.25 × 0.5 cos 15° + 0.25 sin 15° + 0.5 sin 15° − cos 15°)
Fab = 1151.9 N
Now, find P.
P = 1151.9 N (sin 15° + (0.25 + 0.5) cos 15° − 0.25 × 0.5 sin 15°)
P = 1095.4 N