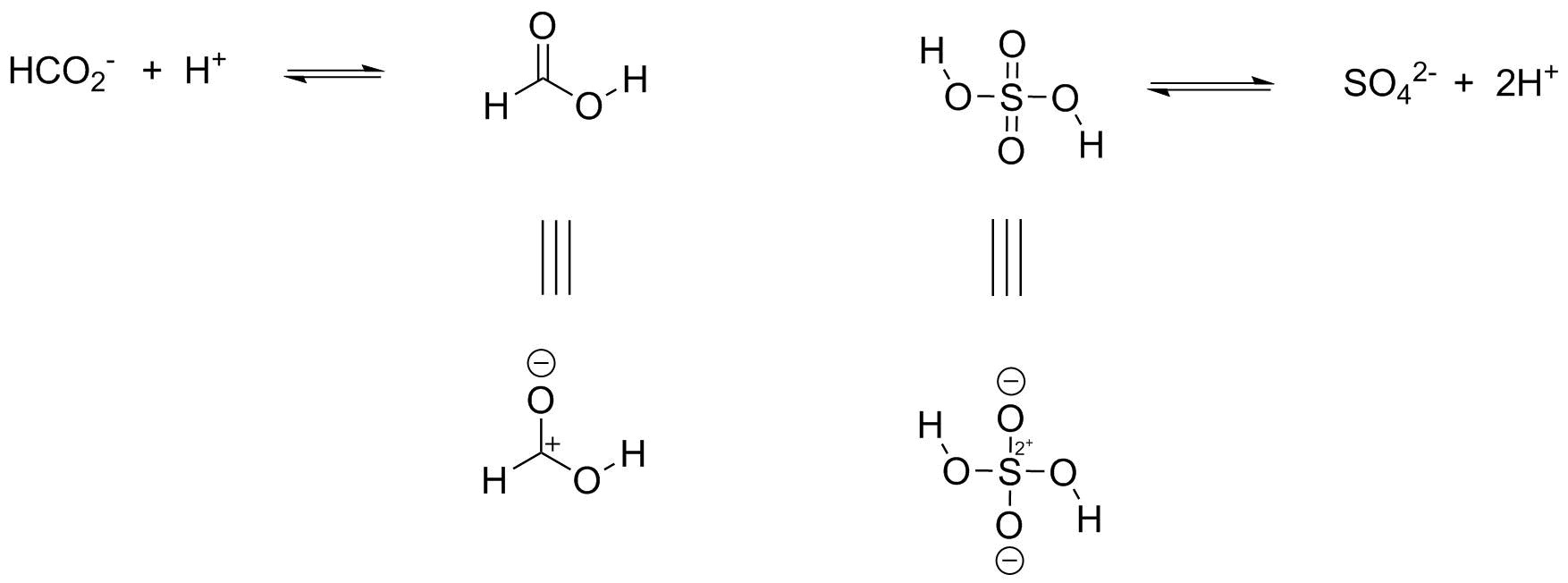
Among formic acid (HCOOH ) and sulfuric acid (H₂SO₄), formic acid is the weak acid. Acidic strength of any acid is the tendency of that acid to loose proton. Among these two acids formic acid has a pKa value of 3.74 greater than that of sulfuric acid i.e. -10. Remember! Greater the pKa value of acid weaker is that acid and vice versa. Below I have drawn the Ionization of both acids to corresponding conjugate bases and protons. The structures below with charges are drawn in order to explain the reason for strength. As it is seen in charged structure of formic acid, there is one positive charge on carbon next to oxygen carrying proton. The electron density is shifted toward carbon as it is electron deficient and demands more electron hence, attracting electron density from oxygen and making the oxygen hydrogen bond more polar. While, in case of sulfuric acid it is depicted that Sulfur attached to oxygen containing proton has 2+ charge, means more electron deficient as compared to carbon of formic acid, hence, more electron demanding and strongly attracting electrons from oxygen and making the oxygen hydrogen bond very polar and highly ionizable.
Correct Answer: <span>(2) The mixture is homogeneous and cannot be separated by filtration.
Reason: Solution is said to be homogenous, if it contains single phase. In present case, salt and water, when dissolved in water forms a single phase. Hence, it is referred as homogenous solution. Also, individual components of homogenous system cannot be separated by the process of filtration. </span><span />
An inter-molecular power is basically an alluring power between neighboring particles. There are three regular sorts of inter-molecular power: lasting dipole-dipole powers, hydrogen bonds and van der Waals' powers.
Answer: -
A) 3.59
Explanation: -
The number of significant figures in 2.06 = 3
The number of significant figures in 1.743 = 4
The number of significant figures in 1.00 = 3
During multiplication of significant figures, the number of significant figures in the answer would be the smallest value that is 3 in this case.
2.06 x 1.743 x 1.00 = 3.5908.
The answer to 3 significant figures is
2.06 x 1.743 x 1.00 = 3.59
Thus, Andi when performs the calculation that is shown below.
(2.06)(1.743)(1.00)
the answer be reported using the correct number of significant figures as A) 3.59
Cross breeding plants to make better apples