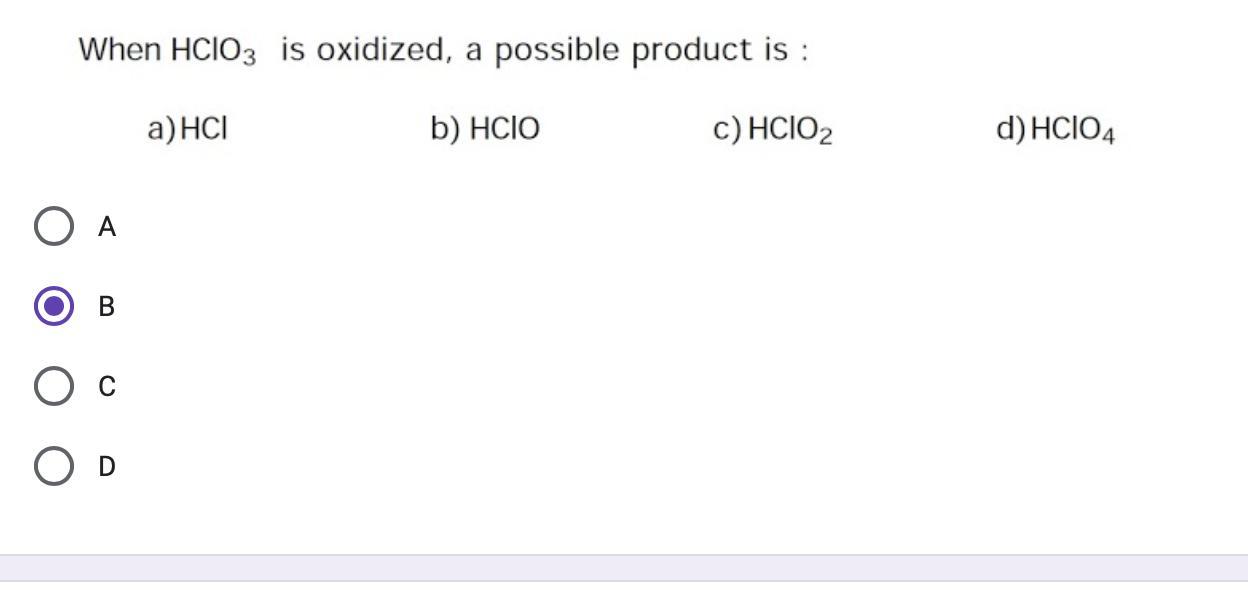
Answer:
What Affects Reaction Rate?
The purpose of this lab was to see how temperature and particle size affects reaction rate. The first hypothesis is if you increase the temperature of a reaction, then the reaction rate will increase because particles experience more collisions at higher temperatures.The second hypothesis is if you decrease the particle size of a reactant, then the reaction rate will increase because more of the reactants’ molecules will contact each other. The independent variables are particle size and temperature. The dependent variable is reaction rate.
Materials
250 mL graduated cylinder
Thermometer
Water
Timer
Four 250 mL beakers
Seven 1,000 mg effervescent tablets
Two pieces of filter paper
600 mL beaker
Ice
Hot plate
Procedure
Step 1:Gather Materials
Variation of Temperature
Step 2:Measure the Reaction Rate at ≈ 20°C (Room Temperature)
a) Using a graduated cylinder, fill a 250 mL beaker with 200 mL of water.
b) Measure the temperature of the water and record it in the correct row of Table A.
c) Reset the timer. Start the timer as you place a full tablet into the beaker.
d) Record the reaction time on the Data Sheet in the correct row of Table A.
e) Compute the reaction rate to the nearest mg/L/sec. Record it in the last column of Table A. Measure the Reaction Rate at ≈ 40°C
Step 3:Repeat Step 2, heating the water to approximately 40°C using a hot plate during sub-step a. Measure the Reaction Rate at ≈ 65°C
Step 4:Repeat Step 2, heating the water to approximately 65°C using a hot plate during sub-step a. Measure the Reaction Rate at ≈ 5°C
Step 5:Repeat Step 2, chilling the water to approximately 5°C inside an ice bath during sub-step a. (To create an ice bath, place 100 mL of ice and 100 mL of water in a 600 mL beaker of ice water and wait until the temperature reaches approximately 5°C. To save time, you may wish to set up the ice bath, using an additional 250 mL beaker, while working on Step 4.)
Variation of Particle Size
Step 6:Measure the Reaction Rate for a Full Tablet
a) Using a graduated cylinder, fill a 250 mL beaker with 200 mL of water.
b) Reset the timer. Start the timer as you place the tablet in the beaker.
c) Record the reaction time on the Data Sheet in the appropriate row of Table B.
d) Compute the reaction rate to the nearest mg/L/sec. Record it in the last column of Table B.
Step 7:Measure the Reaction Rate for a Partially Broken Tablet
Repeat Step 6, but this time break the tablet into eight small pieces on a piece of filter paper. Make sure to place all of the pieces into the beaker at the same time.
Step 8:Measure the Reaction Rate for a Crushed Tablet
Repeat Step 6, but this time crush the tablet into tiny pieces on a piece of filter paper. Make sure to place all of the pieces into the beaker at the same time.
Step 9: Dispose of all samples according to your teacher’s directions.
Measured Reaction Temperature (°C)
Mass of Tablet (mg)
Volume of Water (L)
Reaction Time (s)
Reaction Rate (mg/L/s)
≈20°C
24
1,000
0.2
34.2
146.2
≈40°C
40
1,000
0.2
26.3
190.1
≈65°C
65
1,000
0.2
14.2
352.1
≈5°C
3
1,000
0.2
138.5
36.1
Relative Particle Size (Small, Medium, Large)
Mass of Tablet (mg)
Volume of Water (L)
Reaction Time (s)
Reaction Rate (mg/L/s)
Full Tablet
large
1,000
0.2
34.5
144.9
Broken Tablet
medium
1,000
0.2
28.9
173.0
Crushed Tablet
small
1,000
0.2
23.1
216.5
The data in the first table show that as the temperature increases the reaction time decreases and in turn the reaction rate increases. The data supported the hypothesis that as temperature increases reaction rate will also increase. The second table shows that as the particle size decreases the reaction time increases because there is more surface area when the particles are smaller. The data in the second table supported the second hypothesis that as particle size decreases the reaction rate will increase because there will be more contact in the molecules. Possible source of error would be an error in stopping the timer in time or chips in the tablets. To improve this lab it could be done with different types of reactions or different temperature or different particle sizes.
Explanation: