Explanation :
Edwin Hubble used a telescope to discover the movement of galaxies in 1929. He was an American Astronomer.
The telescope invented by him is called a Hubble telescope. It has a 2.4-meter mirror.
He gives the relation between the distance to a galaxy and its recessional velocity. This is known as the Hubble law. The relation is as follows :

where,
 is recessional velocity.
is recessional velocity.
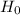 is Hubble constant.
is Hubble constant.
 is distance.
is distance.
<em>So, the given statement is True.</em>
Answer:
He's 3 miles west of school.
Explanation:
He went 5 miles up and 5 miles down which means that he really didn't go up or down. In between that, he went 3 miles west so if the 5 milers don't count, this puts him at 3 miles west of school.
Well gravity is 9.8m/s so i would assume 19.6m/2 is the velocity
Answer:
a. 05cm from x axis
b. 8cm from x axis
Explanation:
If the net magnetic field is zero and the currents are in the same direction then the thanks point is between the currents i1 and i2 as show in the attachment below
a. Given that i1= 5A and i2=3A
Let assume the null point is xcm from current i1, then the null point will be (4-x)cm from current i2 since the total length is 4cm.
Now the magnetic field of the current i1 from the null point= to magnetic field of current i2 from the null point
B1=B2
μi1/2πx=μi2/2π(4-x)
i1/x=i2/(4-x)
5/x=3/(4-x)
20-5x=3x
8x=20
8x=2.5cm
since from the left of x axis is 2cm, then the null point is 2.5-2 which 0.5cm from the origin x axis.
The null point is 0.5cm from the origin x axis
b. If both current are flowing in opposite direction, the null point lies outside of the current.
Then with same analysis let assume the first current i1 is xcm from the null point and since the total length is 4cm the second current i2 will be (x-4)cm from the null point.
Also the magnetic field of the current i1 from the null point = to magnetic field of current i2 from the null point
B1=B2
μi1/2πx=μi2/2π(x-4)
i1/x=i2/(x-4)
5/x=3/(x-4)
5x-20=3x
2x=20
x=10cm.
This shows that the distance of the null point from current i1 is 10cm and the current i1 is 2cm from the x axis, then the null point is 10-2=8cm from the origin x axis.
The null point is 8cm from the x axis.
Check the attachment to see the diagram of the current and the null points