Answer:
Glycogen in an important storage polysaccharide found in animal tissues.
Explanation:
Full question:
Glycogen ________
A) forms the regulatory molecules known as enzymes
B) serves as a structural component of human cells
C) helps to protect vital organs from damage
D) is an important storage polysaccharide found in animal tissues
E) contains the genetic information found in cells
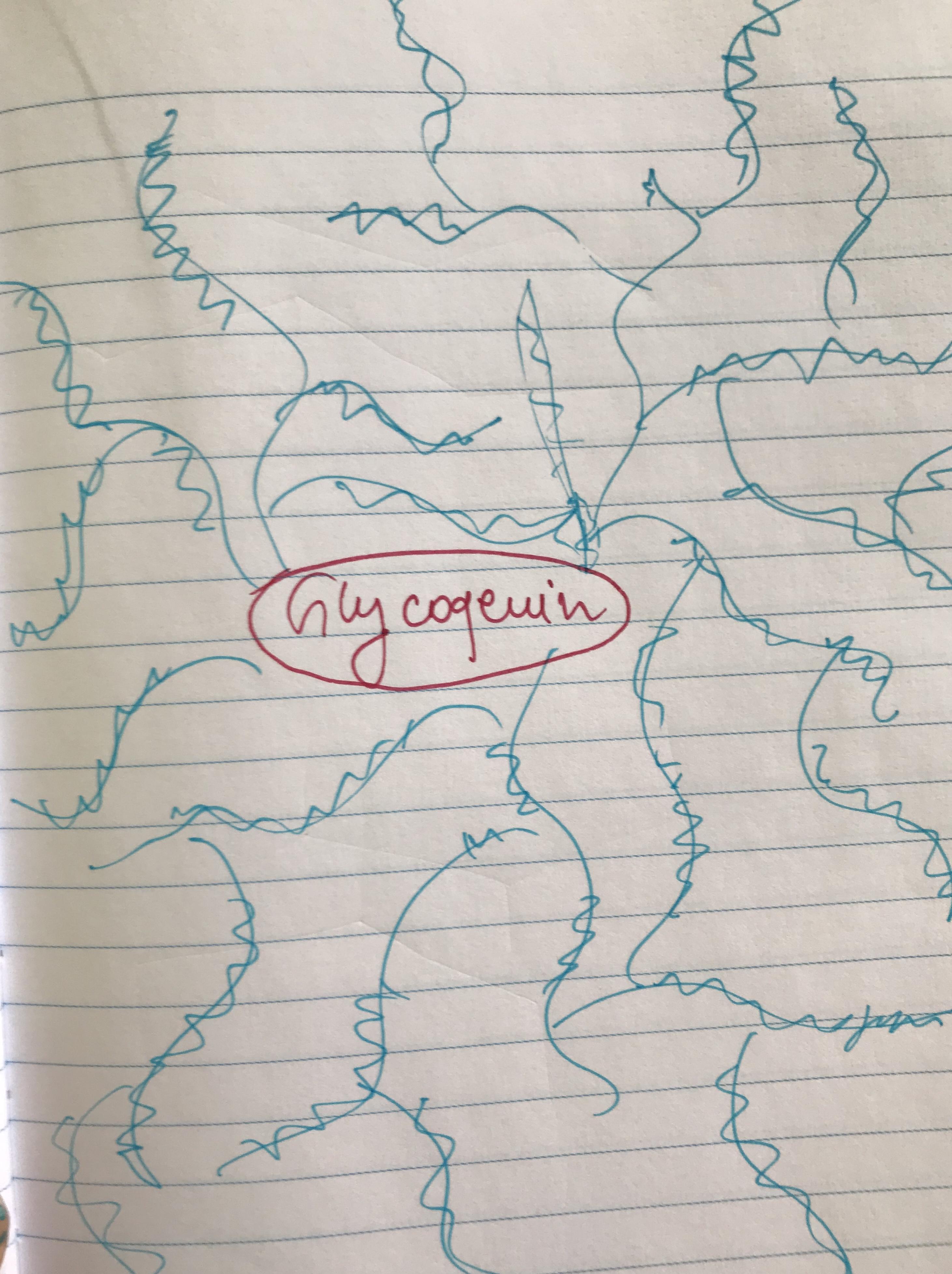
Glycogen is a complex polysaccharide of glucose founded in humans, animals, fungi and even bacteria. In humans, the glycogen is made and stored in liver cells. In the center on glycogen molecule, there is a single protein called Glycogenin. It is a center of a big flower made of glucose molecules (please refer to the scheme attached - Glycogenin is red and the blue lines are glucose chains). Glycogen is also stored in skeletal muscle, red and white blood cells, in glial brain cells and kidneys but in a smaller amounts. It can be found in the placenta in pregnant women where it serves as a nutrient storage for embryo. In an adult, the liver weighs 1,5 kg and glycogen weighs about 120g in such a liver. After a meal, the level of sugar is rising and the insulin is being secreted. Insulin is a tool by which sugar is being delivered to the cells, like a food delivery. During this period, glycogen is being synthesized in the liver out of glucose residues. When the meal is digested, the sugar level is back to normal. When more energy is needed, glycogen from the liver is broken down by glycogen phosphorylase and the new sugar is released into the bloodstream.
Answer:
sorry
Explanation:
I don't know the answer this is really confusing but I am really sorry you have to do this.
The pairs are:
K, Kr - Same period
Be, Mg - Same group
Ni, Tc - Both are transition metals
B, Ge - Both are metaloids
Al, Pb - Both form inert oxides