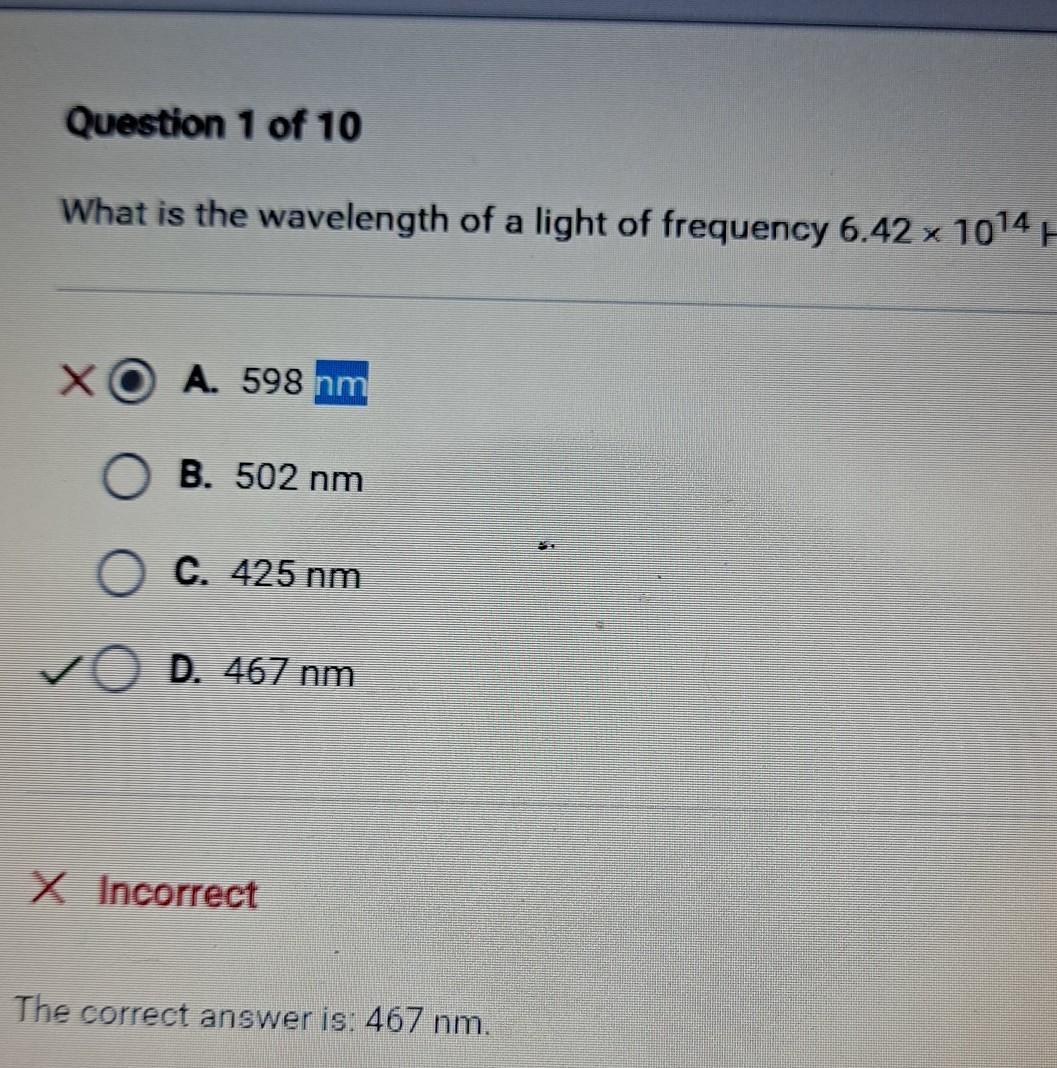
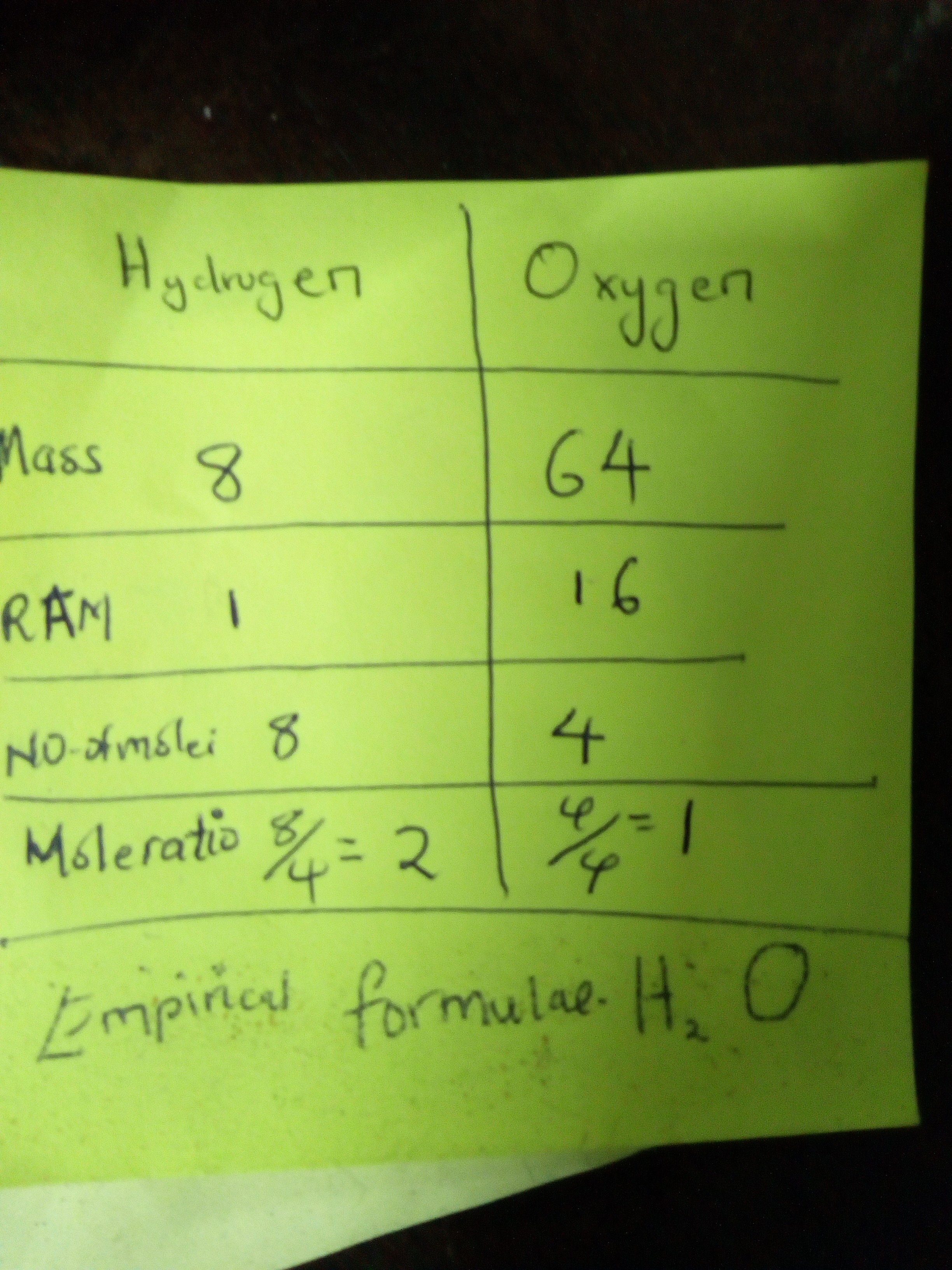
Explanation:
Question 1:
We have to use the aid of the periodic table to solve this problem and decipher some of the species.
The number of protons is the number of positively charged particles. It the same as the atomic number.
Number of electrons is the number of negatively charged particles.
Neutrons do not carry any charges.
Mass number = number of protons + number of neutrons
Solving:
Symbol #atomic #protons #electrons #neutrons mass# atomic mass
Fe 26 26 26 30 56 55.8
As³⁺ 33 33 36 42 75 74.9
Ag 47 47 47 61 108 107.9
I¹⁻ 53 53 54 74 127 126.9
K 19 19 19 20 39 39.1
learn more:
Atomic number brainly.com/question/2057656
Question 2:
An ion is an atom that has either lost or gained electron.
A positively charged ion is one that has lost electrons and the number of protons is now more. They are called cation
A negatively charged specie is one that has gained electrons and the number of electrons are now more. They are called anions.
Mass number is the superscript = Number of protons + number of neutrons
Atomic number = number of protons = number of electrons for neutral atoms
Species:
₇¹⁴N³⁻
Number of protons = 7
Number of electrons = 7 + charge = 7+ 3 = 10
Number of neutrons = 14 - 7 = 7
¹¹²₄₈Cd²⁺
Number of protons = 48
Number of electrons = 48 -2 = 46
Number of neutrons = 112 - 48 = 64
⁶⁴₂₉Cu
Number of protons = 29
Number of electrons = 29
Number of neutrons = 64 - 29 = 35
¹¹⁹₄₄Sn⁴⁺
Number of protons = 44
Number of electrons = 44 - 4 = 40
Number of neutrons = 119 - 44 = 75
¹⁹₉F⁻
Number of protons = 9
Number of electrons = 9+ 1 = 10
Number of neutrons = 19 - 9 = 10
learn more:
Number of protons, neutrons and electrons brainly.com/question/2757829
Question 3:
Mass number
The number 79 in Se - 79 specifies the mass number of the element. It suggests that Se is the number 34 element on the periodic table.
- The periodic table arranges elements based on their atomic number.
- The atomic number is the number of protons in an atom
- Every atoms has a unique atomic number which makes them different from other atoms.
- In neutral atoms, the atomic number is the same as the number of protons and the number of electrons in an atom.
- The mass number is the number of protons plus neutrons of an atom.
learn more:
Mass of proton brainly.com/question/4999318
Question 4:
Number of protons = 14
Atomic structure:
²⁸₁₄Si
- In this atom designated above Si is the symbol of Silicon, the fourteenth element in the periodic table.
- The superscript 28 is the mass number of the atom
- The mass number is the number of protons and neutrons in the nucleus of the atom.
- It is also called the nucleon number because protons and neutrons are nucleons.
- The subscript 14 is the atomic number.
- It is the number of protons in the atom.
- For neutral atoms, it is also the number of electrons
learn more:
Number of protons, neutrons and electrons brainly.com/question/2757829
Question 5:
The total number of subatomic particles = 289
Structure is ²⁰⁷₈₂Pb
- The subatomic particles in an atom are protons, neutrons and electrons
- Protons are the positively charged particles in an atom
- Neutrons have no charge
- Electrons carry negative charges
In the atom above, since it is neutral, the number of protons = number of electrons
Number of protons = atomic number
This is the subscript;
Number of protons = 82
Number of electrons = 82
Number of neutrons = mass number - atomic number
Mass number is the superscript
Number of neutrons = 207 - 82 = 125
The total number of subatomic particles = number of protons + number of neutrons + number of electrons
Total number of subatomic particles = 82 + 82 + 125 = 289
learn more:
protons, neutrons and electrons brainly.com/question/2757829
Question 6:
29
Given parameters :
Number of neutrons = 31
Mass number = 59
Unknown:
Atomic number = ?
Solution:
The mass number of an atom is the number of protons and neutrons it contains.
The mass of an atom is concentrated in the nucleus and this is where we derive the mass number from;
Mass number = number of protons + number of neutrons
Number of protons = 59 - 31 = 28
The number of protons in an atom is the same as the atomic number.
Atomic number = 28
learn more:
Atomic number brainly.com/question/2057656
Question 13 remainder;
When sodium loses an electron, the number of protons will be more than that of electrons
It then becomes an ion;
Number of protons = 11
Number of electrons = 11 - 1 = 10 Charge = 11 - 10 = 1+
With a loss of one electron, sodium will now resemble Ne more closely because it has a stable configuration.
learn more
lon brainly.com/question/4670413
#learnwithBrainly