Answer:
1- 1.54 mol.
2- 271.9 kPa.
3- Yes, the tires will burst.
4- 235.67 kPa.
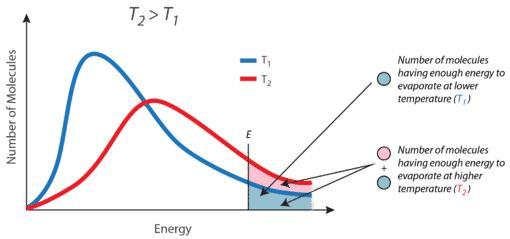
5- As, the temperature increased, the no. of molecules that has minimum kinetic energy increases as shown in image 1 that represents the Maxwell’s Distribution of Speeds of molecules. "Kindly, see the explanation and the attached images".
Explanation:
<u><em>Q1- How many moles of nitrogen gas are in each tire? </em></u>
- To calculate the no. of moles of nitrogen gas in each tire, we can use the general law of ideal gas: PV = nRT.
where, P is the pressure of the nitrogen gas (P = 247.0 kPa/101.325 = 2.44 atm),
V is the volume of the nitrogen gas (V = 15.2 L),
n is the no. of moles of the nitrogen gas (n = ??? mole),
R is the general gas constant (R = 0.082 L.atm/mol.K),
T is the temperature of the nitrogen gas (T = 21°C + 273 = 294 K).
∴ n = PV/RT = (2.44 atm)(15.2 L)/(0.082 L/atm/mol.K)(294.0 K) = 1.54 mol.
<u><em>Q2: What would the maximum tire pressure be at 50 degrees C? </em></u>
- Now, the temperature is raised to be 50°C (T = 50°C + 273 = 323 K).
- The pressure can be calculated using the general gas law: PV = nRT.
∴ P = nRT/V = (1.54 atm)(0.082 L/atm/mol.K)(323.0 K)/(15.2 L) = 2.68 atm = 271.9 kPa.
<em>Q3: Will the tires burst in Spokane? Explain.</em>
- <em>Yes, the tires will burst because the internal pressure be 271.9 kPa that exceeds 270 kPa, the pressure above which the tires will burst. </em>
<u><em>Q4: If you must let nitrogen gas out of the tire before you go, to what pressure must you reduce the tires before you start your trip? (Assume no significant change in tire volume.) </em></u>
- To get the pressure that we must begin with:
Firstly, we should calculate the no. of moles at:
T = 55°C + 273 = 328 K,
Pressure = 270 kPa (the pressure above which the tires will burst). (P =270 kPa/101.325 = 2.66 atm).
V = 15.2 L, as there is no significant change in tire volume.
∴ n = PV/RT = (2.66 atm)(15.2 L)/(0.082 L.atm/mol.K)(328 K) = 1.5 mol.
- <em>1.5562 moles of N₂ in the tires will give a pressure of 270 kPa at 55°C, so this is the minimum moles of N₂ that will make the tires burst. </em>
- <em>Now, we can enter this number of moles into the original starting conditions to tell us what pressure the tires will be at if we start with this number of moles of N₂. </em>
P = ???
V = 15.6 L.
n = 1.5 mol
T = 21°C + 273 = 294.0 K
R = 0.0821 L.atm/mol.K.
∴ P = nRT/V = (1.5 mol x 0.082 x 294.0 K) / (15.6 L) = 2.2325 atm = 235.67 kPa.
So, the starting pressure needs to be 235.67 kPa or just under in order for the tires not to burst.
<u><em>Q5: Create a drawing of the tire and show a molecular view of the air molecules in the tire at 247 kpa vs the molecular view of the air molecules after the tires have been heated. Be mindful of the number of molecules that you use in your drawing in the before and after scenarios. Use a caption to describe the average kinetic energy of the molecules in both scenarios.</em></u>
<u><em /></u>
- <em>As, the temperature increased, the no. of molecules that has minimum kinetic energy increases as shown in “image 1” that represents the Maxwell’s Distribution of Speeds of molecules. </em>
- <em>The no. of molecules that possess a critical K.E. of molecules increases due to increasing the temperature activate the motion of molecules with high velocity as </em>
- <em>(K.E. = 3RT/2), K.E. directly proportional to the temperature of the molecules (see image 2). </em>
- <em>Also, the average speed of molecules increases as the K.E of the molecules increases (see image 3). </em>