Answer:
Explanation:
From the information given:
We can say that the rate of reaction is utilized to decide the reaction speed, and it is subject to the rate constant.
The power of concentration related to the rate equation can be said to be the order of the reaction.
For zero-order reaction: it is the reaction whose rate is free and not dependent on the reactant's concentration.
Concentration-time reaction is given as;
where;
t = time
k = rate constant
A_o = initial concentration of reactant
Thus, the plot between [A] and t needs to be a straight line.
On the other hand, The First order reaction is the reaction whose rate is straightforwardly corresponding to the reactant's concentration.
Its concentration-time relation can be expressed as,
t = time
k = rate constant
[A_o] = initial concentration of the reactant
Then, the plot between [A] & t requires to be a straight line.
Presently, the plot between ln([A]) vs time appears to be in a straight line with the slope of the line equivalents to the rate constant (k).
hence,
slope = -k
Then;
Now; to discover the activation energy we need to utilize Arrhenius relation which is given as,
From the above equation;
k_o = arrhenius parameter
Ea = activation energy
R = 8.314 J/mol.K
T = temperature in Kelvin
From the data given for concentration vs time;
t[min] Ca(mol/L)
0 2
5 1.6
9 1.35
15 1.1
22 0.87
30 0.7
40 0.53
60 0.35
a)
Since we can deduce the order of the reaction,
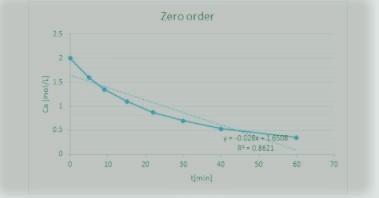
Let's assume that it is a zero-order reaction, Thus, the plot of Ca vs t can be seen in the first image attached below;
From the diagram, it is clear and obvious that it is NOT a straight line.
Thus, we conclude that this order is NOT a zero-order.
However, let also assume that order is first order,
Now, from the second diagram showing the plot of ln(Ca) vs t, we can deduce that it is a straight line which implies that it is the order of the reaction is first order.
Therefore, the equation for the first order is given as;
Recall that: the slope of the ln(A) v/s time will result in a rate constant,
Thus, from the graph we have;
slope = -0.0289 mol/L.min and:
slope = - k
Hence;
rate constant = k = 0.0289 mol/L.min and the order = 1st order
b)
On the off chance that we need to take more data points, we will like to take the data point in the scope of 40 min to 60 min time interval, on the grounds that the significant deviation is seen there. more information focuses will imply more accuracy.