NOTICE: There are attachments attached to this answer that is mentioned in each paragraph!
We frequently see DNA replication as a well-organized, methodical process, much like a production line. It's not. For the DNA polymerase to read it, the DNA must not lie in a straight line and must be in motion, not static. It is curled and twisted. First, realize that this is a molecular issue. Bacteria lack neurons, cannot "think," and cannot make decisions. Everything is a chemical reaction, and chemical reactions frequently depend on one concentration's osmotic pressure being higher than another, both inside the cell and outside. Even at that level, there is a lot of "nothing," albeit nothing is empty. For a better understanding of the environment, picture a cell as a large room filled with balls of all sizes. Each ball has a unique form and isn't spherical; some balls fit together flawlessly while others don't. This is the easiest way to conceptualize the universe of molecular chemistry. There is never "nothing" other than in space when you push your way through layers upon layers of free oxygen, free nitrogen, helium, carbon dioxide, hydrogen dioxide, argon, methane, etc. when you walk through what we term "air."
Replication mistakes happen. Sometimes DNA polymerase enzymes add the incorrect nucleotide, too many, or too few nucleotides to a sequence. Or the DNA polymerase gets looped on the same strand, adding a few codons in a repetition before the strand slips away and it continues, or there is a tangle of crossing DNA and it jumps to the incorrect thread and back (or never), etc. In order to ensure that the bases added to a developing strand are appropriately matched with their complements, DNA polymerase enzymes are quite picky about the nucleotides they choose to use. However, these enzymes do make errors. Specifically, at a rate of around 1 per 100,000 nucleotides. Doesn't sound like much, but since each diploid cell has 6 billion base pairs, there are around 120,000 errors every cell division.
Ribosomal errors during transcription can even change the synthesis of the DNA polymerase proteins, leading to the creation of a mechanism that is already faulty. Since not all ribosomes are created equally, ribosome quality is important. Bacterial mutation rates are greater because bacterial ribosomes are less accurate and produce a lot more errors than human ribosomes. That is a benefit for simple, quickly reproducing life, which is also very vulnerable to the stresses of the molecular chemical environment. Unlike most more complex forms of life, which also have DNA repair mechanisms incorporated into the DNA polymerase, humans have these mechanisms. Some of these processes can often correct mistakes as soon as they are produced, while others can do so over time.
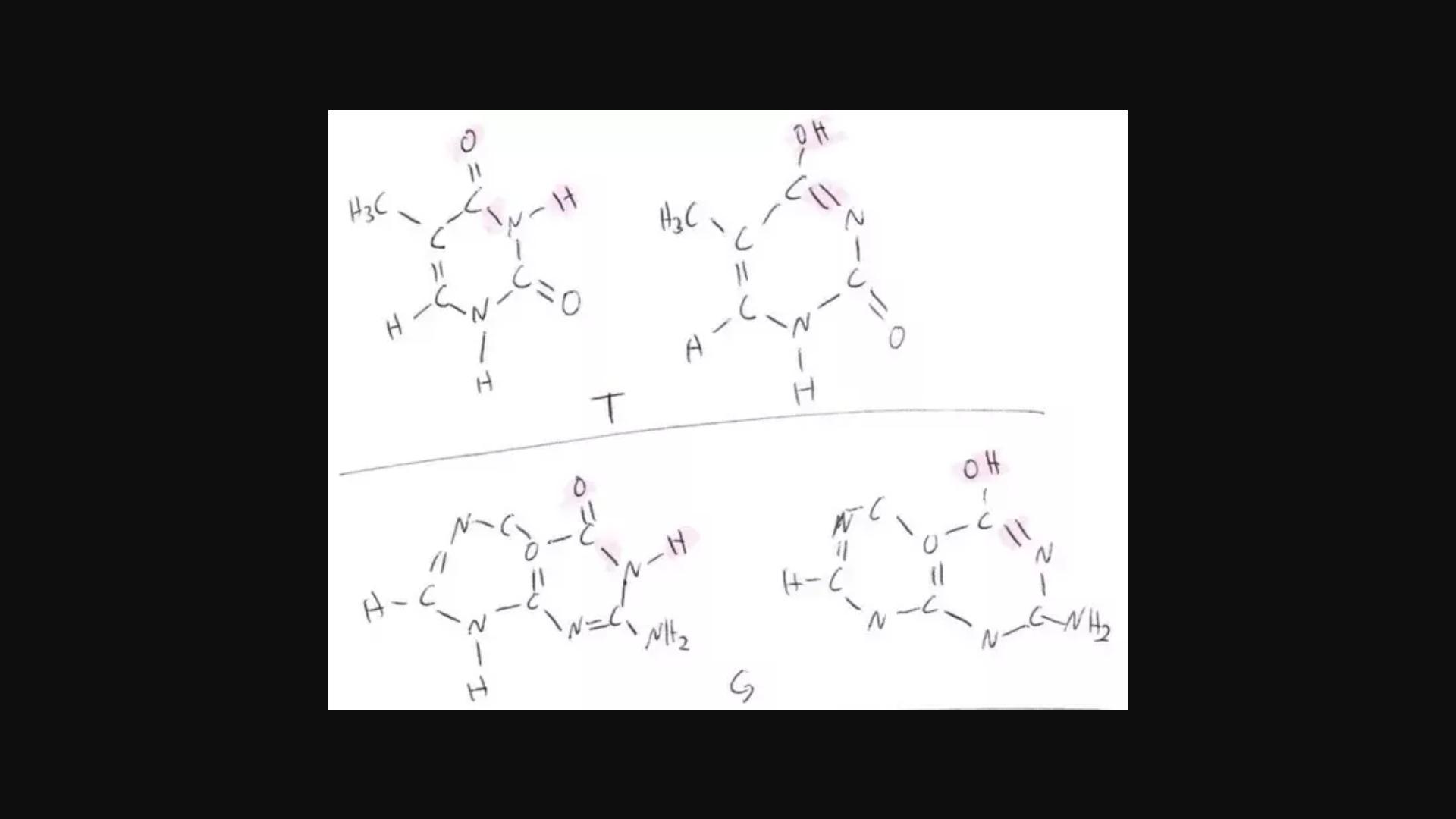
Regarding the pairing of C and T, etc. There are a number of balls in that room that, at first glance, appear to be identical, but if you compare two closely, you could find that one bump is larger or is located incorrectly even if it appears to be same. Proton shift is one such. Here are two instances using the purine guanine (G) and the pyrimidine thymine (T) (G). Please excuse my handwriting; I learned to type at a very young age. Shifted on the right, normal on the left. NA polymerase (as do most more complex forms of life) frequently correct mistakes as soon as they are made and others that correct mistakes later.
<em>(ATTATCHMENT #1)</em>