Answer:
1.512 m/s
Explanation:
M₁ = 10.5
M₂ = 61.5kg
v₁ = 8.85m/s
v₂ = ?
This question involves conservation of momentum and law of conservation of momentum states that, the total momentum of a system does not change before and after impact and it's always constant.
Momentum = mass * velocity = M*V
total momentum of the system before caught the ball = total momentum after he caught the ball.
M₁V₁ = M₂V₂
10.5 * 8.85 = 61.5 * V₂
V₂ = 92.925 / 61.5
V₂ = 1.5109m/s ≈ 1.512m/s
B,I'm pretty sure that's what it is
Answer:
Distance will be 49.34 m
Explanation:
We have given wavelength 
Diameter of the antenna d = 2.7 m
Range L = 7.8 km = 7800 m
We have to find the smallest distance hat two speedboats can be from each other and still be resolved as two separate objects D
We know that distance is given by 
So distance D will be 49.34 m
Answer:
Answer: Science and technology are relatively modern developments. For most of the past 50,000 years of civilization, there was religion, dogma, ritual an tradition. These kept civilization safe but prevented growth.
After the modern scientific method was developed during the Rennaisance, civil action took off.
Health, as expressed as life expectancies, has increased massively. Population growth has increased to levels thought impossible before. But yes, the environment has suffered.
Explanation :
Edwin Hubble used a telescope to discover the movement of galaxies in 1929. He was an American Astronomer.
The telescope invented by him is called a Hubble telescope. It has a 2.4-meter mirror.
He gives the relation between the distance to a galaxy and its recessional velocity. This is known as the Hubble law. The relation is as follows :

where,
 is recessional velocity.
is recessional velocity.
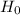 is Hubble constant.
is Hubble constant.
 is distance.
is distance.
<em>So, the given statement is True.</em>