Answer:
☟
Explanation:
Fuels made from oil mixtures containing large hydrocarbon molecules are not efficient as they do not flow easily and are difficult to ignite. Crude oil often contains too many large hydrocarbon molecules and not enough small hydrocarbon molecules to meet demand. This is where cracking comes in.
Cracking allows large hydrocarbon molecules to be broken down into smaller, more useful hydrocarbon molecules. Fractions containing large hydrocarbon molecules are heated to vaporise them. They are then either:
heated to 600-700°C
passed over a catalyst of silica or alumina
These processes break covalent bonds in the molecules, causing thermal decompositionreactions. Cracking produces smaller alkanesand alkenes (hydrocarbons that contain carbon-carbon double bonds). For example:
hexane → butane + ethene
C6H14 → C4H10 + C2H4
Some of the smaller hydrocarbons formed by cracking are used as fuels, and the alkenes are used to make polymers in plastics manufacture. Sometimes, hydrogen is also produced during cracking.
Fractional distillation of crude oil
Fractional distillation separates a mixture into a number of different parts, called fractions.
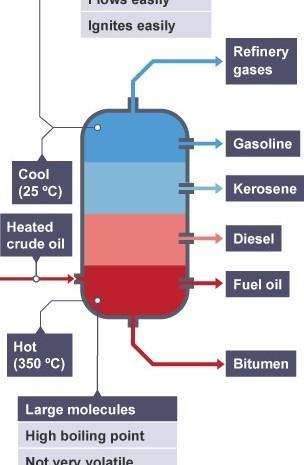
A tall fractionating column is fitted above the mixture, with several condensers coming off at different heights. The column is hot at the bottom and cool at the top. Substances with high boiling points condense at the bottom and substances with lower boiling points condense on the way to the top.
Crude oil is a mixture of hydrocarbons. The crude oil is evaporated and its vapours condense at different temperatures in the fractionating column. Each fraction contains hydrocarbon molecules with a similar number of carbon atoms and a similar range of boiling points.
Oil fractions
The diagram below summarises the main fractions from crude oil and their uses, and the trends in properties. Note that the gases leave at the top of the column, the liquids condense in the middle and the solids stay at the bottom.
As you go up the fractionating column, the hydrocarbons have:
lower boiling points
lower viscosity (they flow more easily)
higher flammability (they ignite more easily).
Other fossil fuels
Crude oil is not the only fossil fuel.
Natural gas mainly consists of methane. It is used in domestic boilers, cookers and Bunsen burners, as well as in some power stations.
Coal was formed from the remains of ancient forests. It can be burned in power stations. Coal is mainly carbon but it may also contain sulfur compounds, which produce sulfur dioxide when the coal is burned. This gas is a cause of acid rain. Also, as all fossil fuels contain carbon, the burning of any fossil fuel will contribute to global warming due to the production of carbon dioxide.