The circuit is no longer closed.
Given the Hubble's constant, the approximate age of the universe is 5.88 × 10⁹ Years.
Given the data in the question;
Hubble's constant; 
Age of the universe; 
We know that, the reciprocal of the Hubble's constant ( 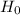 ) gives an estimate of the age of the universe (
) gives an estimate of the age of the universe (  ). It is expressed as:
). It is expressed as:

Now,
Hubble's constant; 
We know that;

so
![1\ Million\ light\ years = [9.46 * 10^{15}m] * 10^6 = 9.46 * 10^{21}m](https://tex.z-dn.net/?f=1%5C%20Million%5C%20light%5C%20years%20%3D%20%5B9.46%20%2A%2010%5E%7B15%7Dm%5D%20%2A%2010%5E6%20%3D%209.46%20%2A%2010%5E%7B21%7Dm)
Therefore;

Now, we input this Hubble's constant value into our equation;

Therefore, given the Hubble's constant, the approximate age of the universe is 5.88 × 10⁹ Years.
Learn more: brainly.com/question/14019680
Answer:
The Short Answer: Sunlight reaches Earth's atmosphere and is scattered in all directions by all the gases and particles in the air. Blue light is scattered more than the other colors because it travels as shorter, smaller waves. This is why we see a blue sky most of the time.
Explanation:
Able to be hammered or pressed permanently out of shape without breaking or cracking.
Answer:
I would say the answer is Non-Biodegradable......eg, plastics, rubber, metals,etc.
HOPE THIS HELPS.