Answer:
Technician B
Explanation:
When working on vehicles which are equipped with a catalytic coverter, the best practice of asphyxiating someone is to use carbon monoxide. This implies that you need an extraction sytem. Therefore, technician A is wrong by assuming no need of the system. In conclusion, technician B is correct.
Answer:
2102.1 m
Explanation:
Temperature at the equator = 0⁰
Radius of the earth = 6.37x10⁶
Required:
We how to find out what the clearance between tape and ground would be if temperature increases to 30 degrees.
Final temperature = ∆T = 303-273 = 30
S = 11x10^-6
The clearance R = Ro*S*∆T
=6.37x10⁶x 11x10^-6x30
= 2102.1m
Or 2.102 kilometers
Thank you
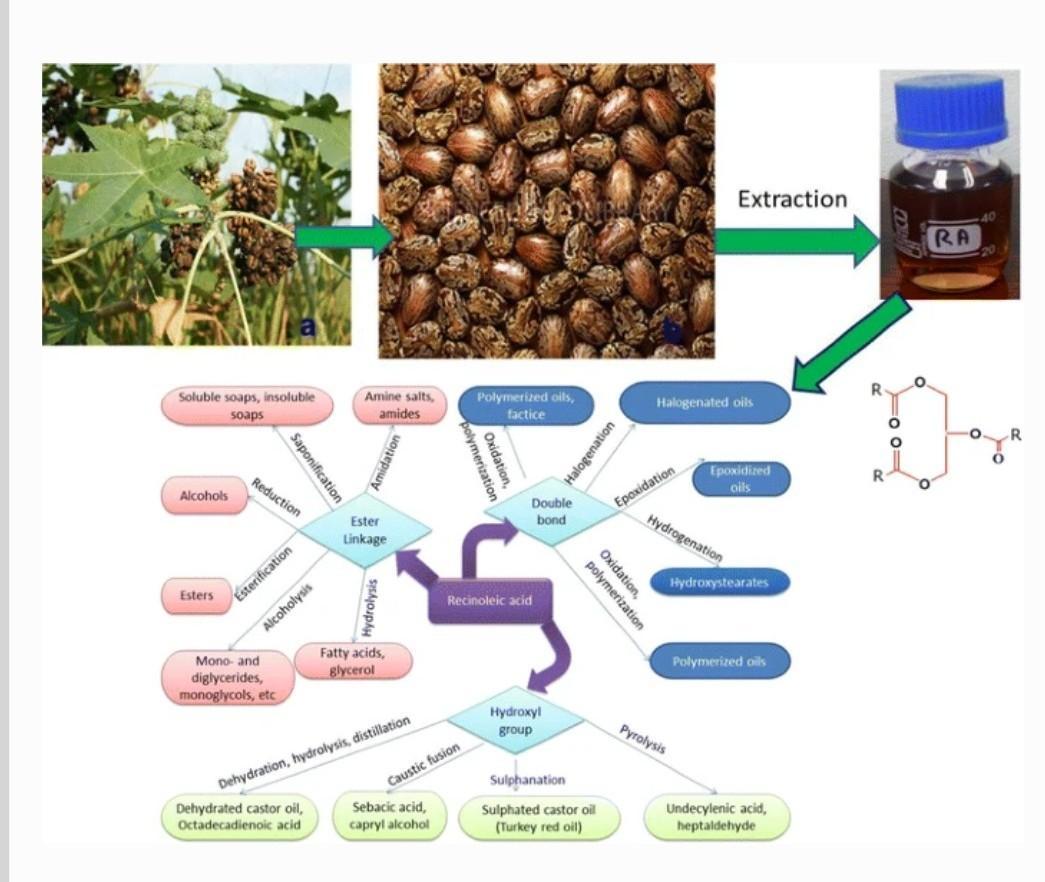
Castor oil is increasingly becoming an important bio-based raw material for industrial applications. The oil is non-edible and can be extracted from castor seeds from the castor plant belonging to the family Euphorbiaceae. The oil is a mixture of saturated and unsaturated fatty acid esters linked to a glycerol. The presence of hydroxyl group, a double bond, carboxylic group and a long chain hydrocarbon in ricinoleic acid (a major component of the oil), offer several possibilities of transforming it into variety of materials. The oil is thus a potential alternative to petroleum-based starting chemicals for the production of materials with variety of properties. Despite this huge potential, very little has recently been reviewed on the use of castor oil as a bio-resource in the production of functional materials. This review therefore highlights the potential of castor oil in the production of these diverse materials with their projected global market potential. The review gives the background information of castor oil and its geographical availability, the properties and its uses as bio-based resource for synthesis of various materials. The review further highlights on the use of castor oil or ricinoleic acid as a green capping agent in the synthesis of nanomaterials.
Answer:
Explanation:
do you have any other questions besides "tyuuyiopopiouyttrrtrffrlkl,k;;';'l.l"