The universe is "running down," becoming randomly scattered and disorganized, tending to break down into its simple parts. Metals rust, parts wear out, dead organisms decay and turn to dust. The entropy of the universe is increasing.
The Bible provides an answer. A great amount of energy had to be expended to produce such great organization. As one scientist states the problem, energy was not enough. If you are asked to "reduce the entropy" of your room (put it in order), you certainly need to put in some effort (energy). But just adding energy is not enough. You could let loose a bull in your room, and it would expend a great deal of energy; however, that energy would not decrease but would increase the disorder of the room. Therefore, scientists know that to produce order, energy must be intelligently directed. Evolution requires that atoms and molecules assemble themselves into increasing order, but the science of thermodynamics assures us that the entire universe, when left to itself, is converting to disorder. The Apostle Paul referred to this concept when he said through inspiration that the entire earth is "subject to the bondage of decay." The original state required an intelligence to organize the complexities of the universe.
Christians, of course, have an answer to these perplexing questions: God. God is the Creator of the universe, the ultimate source of its energy and order. He created it with no entropy (no disorder). When man sinned, the universe began to increase in entropy (become more and more disordered). For Christians a discussion of energy, thermodynamics, and entropy is inspiring. These subjects invariably point us to the One who created the universe and sustains it by His power. Christians also know that His power will one day totally renew the universe and entropy will have no part in it.
Christians need never be afraid of science. God is in control of the universe and all the laws of the universe operate according to His will and design. Christians should be confident that they have the answers to the perplexing questions of nature.
Part 2
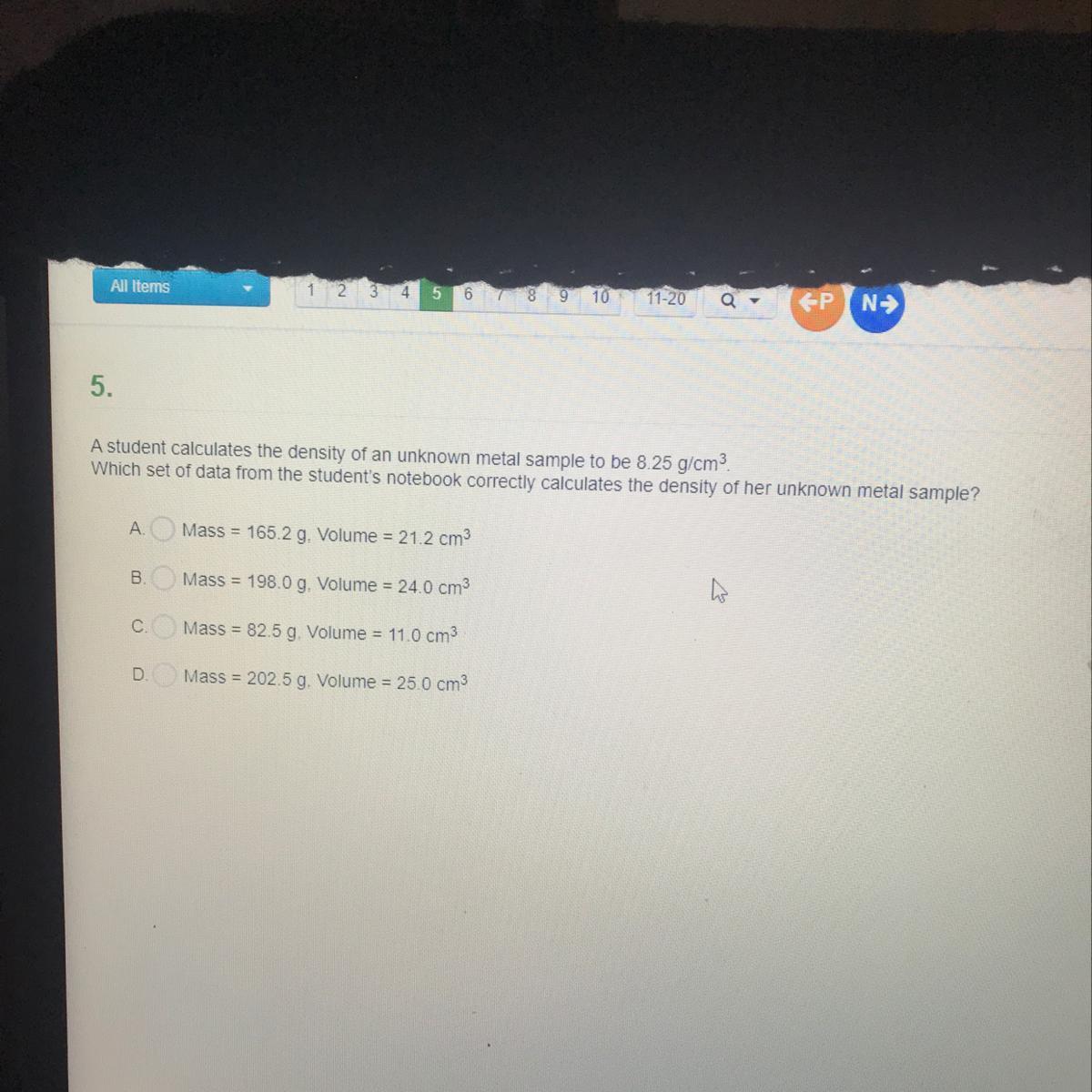
The above attitude allows christians to ignore questions that are difficult and this leads to mental complacency.
"This is gods work. I must not question or touch this". Is their motto.
When christians see difficult questions, such as entropy and creation, that is where they stop thinking and attribute it to god. They become intellectually crippled and refuse to make that one further step. It then halts progress until the shamen can integrate new knowledge into their mantra and dogma.
Science in general, tries to explain and understand and make further progress and additions to human knowledge. Christians will only try to understand what they have been taught and integrate any new knowledge when the older beliefs become untenable.
The Bible provides an answer. A great amount of energy had to be expended to produce such great organization. As one scientist states the problem, energy was not enough. If you are asked to "reduce the entropy" of your room (put it in order), you certainly need to put in some effort (energy). But just adding energy is not enough. You could let loose a bull in your room, and it would expend a great deal of energy; however, that energy would not decrease but would increase the disorder of the room. Therefore, scientists know that to produce order, energy must be intelligently directed. Evolution requires that atoms and molecules assemble themselves into increasing order, but the science of thermodynamics assures us that the entire universe, when left to itself, is converting to disorder. The Apostle Paul referred to this concept when he said through inspiration that the entire earth is "subject to the bondage of decay." The original state required an intelligence to organize the complexities of the universe.
Christians, of course, have an answer to these perplexing questions: God. God is the Creator of the universe, the ultimate source of its energy and order. He created it with no entropy (no disorder). When man sinned, the universe began to increase in entropy (become more and more disordered). For Christians a discussion of energy, thermodynamics, and entropy is inspiring. These subjects invariably point us to the One who created the universe and sustains it by His power. Christians also know that His power will one day totally renew the universe and entropy will have no part in it.
Christians need never be afraid of science. God is in control of the universe and all the laws of the universe operate according to His will and design. Christians should be confident that they have the answers to the perplexing questions of nature.
Part 2
The above attitude allows christians to ignore questions that are difficult and this leads to mental complacency.
"This is gods work. I must not question or touch this". Is their motto.
When christians see difficult questions, such as entropy and creation, that is where they stop thinking and attribute it to god. They become intellectually crippled and refuse to make that one further step. It then halts progress until the shamen can integrate new knowledge into their mantra and dogma.
Science in general, tries to explain and understand and make further progress and additions to human knowledge. Christians will only try to understand what they have been taught and integrate any new knowledge when the older beliefs become untenable.
3
0